A phase II trial of mTORC1/2 inhibition in STK11 deficient non small cell lung cancer
- PMID: 40069402
- PMCID: PMC11897347
- DOI: 10.1038/s41698-025-00838-4
A phase II trial of mTORC1/2 inhibition in STK11 deficient non small cell lung cancer
Abstract
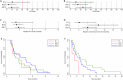
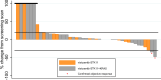
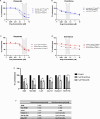
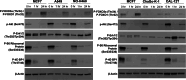
There are no current stratified medicine options for STK11-deficient NSCLC. STK11 loss mediates mTORC activation, GLUT1 up-regulation and increased glycolysis. This metabolic reprogramming might represent a therapeutic vulnerability targetable with mTORC1/2 inhibition. In arm B2 of the National Lung Matrix Trial 54 patients with NSCLC received vistusertib, of which 49 were STK11-deficient (30 with KRAS mutation (B2D), 19 without (B2S)). Objective response (OR) and durable clinical benefit (DCB) rates with 95% credible intervals (CrI) were estimated from posterior probability distributions generated using Bayesian beta-binomial conjugate analysis. In B2D, 2 per-protocol patients obtained OR (estimated true OR rate (95%CrI) 9.8% (2.4-24.3). Estimates of true DCB rate (95%CrI): B2D 24.4% (11.1-42.3), B2S 14.6% (3.6-34.7). Overall, vistusertib cannot be recommended in this context. Longitudinal ctDNA analysis demonstrates enrichment of SMARCA4 mutations post-treatment. In vitro studies show adaptive resistance to mTORC1/2 inhibition via AKT reactivation. (NCT02664935, ISRCTN38344105, EudraCT 2014-000814-73, 10 June 2015).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.M. received research funding from AstraZeneca and speaker bureau fees. Y.S. received an honorarium from AstraZeneca for advisory boards and educational events. A.G. and J.S.p received an honorarium from AstraZeneca for advisory boards. S.P. received consultancy and speaker bureau fees from AstraZeneca. P.J. received funding and consultancy honorarium from AstraZeneca. P.S., D.G. and D.F. received honorariums from AstraZeneca. M.B., D.M.B. and U.M. are employees of Illumina Cambridge Ltd. A.M.F. is a co-inventor on a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987). A.D.B. received research funding from AstraZeneca. C.S. acknowledges grant support and honorarium from AstraZeneca and is an AstraZeneca advisory board member; honorarium from Illumina; is listed as an inventor on a European patent application relating to assay technology to detect tumour recurrence (PCT/GB2017/053289), the patent has been licensed to commercial entities and, under his terms of employment, C.S. is due a revenue share of any revenue generated from such license(s); holds patents relating to targeting neoantigens (PCT/EP2016/059401), identifying patient response to immune checkpoint blockade (PCT/EP2016/071471), determining HLA LOH (PCT/GB2018/052004), predicting survival rates of patients with cancer (PCT/GB2020/050221), identifying patients who respond to cancer treatment (PCT/GB2018/051912), a US patent relating to detecting tumour mutations (PCT/US2017/28013), methods for lung cancer detection (US20190106751A1) and both a European and US patent related to identifying insertion/deletion mutation targets (PCT/GB2018/051892) and is listed as a co-inventor on a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987) and is a named inventor on a provisional patent protection related to a ctDNA detection algorithm. All other authors declare no relevant potential conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

