FAPI radiopharmaceuticals in nuclear oncology and theranostics of solid tumours: are we nearer to surrounding the hallmarks of cancer?
- PMID: 40069442
- PMCID: PMC12014767
- DOI: 10.1007/s12149-025-02022-x
FAPI radiopharmaceuticals in nuclear oncology and theranostics of solid tumours: are we nearer to surrounding the hallmarks of cancer?
Abstract
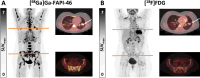
[18F]FDG PET/CT is the most widely used PET radiopharmaceutical in oncology, but it is not exempt of diagnostic limitations. FAPI have emerged as a great tool in the management of several different solid tumours in which [18F]FDG is not able to provide enough information. The aim of this work was to evaluate the available evidence on diagnostic and therapeutic applications of PET/CT with FAPI radiopharmaceuticals. We underwent a non-systematic review focusing in the utility of FAPI radiopharmaceuticals in PET/CT diagnosis and in the treatment of several malignancies. FAPI radiopharmaceuticals present characteristics that can potentially overcome some known diagnostic limitations of [18F]FDG. FAPI radiopharmaceuticals present a high target-to-background ratio (TBR) in many solid tumours such as oesophageal cancer, gastric cancer, pancreatic cancer, hepatic cancer, colorectal cancer, breast cancer, ovarian, cervical cancer, and head and neck cancer. Available evidence suggests the high TBR improves sensitivity and specificity compared to [18F]FDG, especially for the detection of lymphadenopathies and peritoneal metastases, and may improve patient management and radiation treatment planning. Moreover, it is important to underline the potential theranostic application of FAPI radiopharmaceuticals.
Keywords: Cancer; FAPI; Nuclear oncology; PET/CT; Radiopharmaceutical therapy; Theranostic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: KH: Reports personal fees from Bayer, personal fees and other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Fusion, personal fees from Immedica, personal fees from Onkowissen.de, personal fees from Novartis, personal fees from Molecular Partners, personal fees from ymabs, personal fees from Aktis Oncology, personal fees from Theragnostics, personal fees from Pharma15, personal fees from Debiopharm, personal fees from AstraZeneca, personal fees from Janssen. KMP: IPSEN (travel fees), Novartis (consultant, travel fees), Bayer (research funding), GE Healthcare (consultant), Clinician Scientist Stipend from the University Medicine Essen Clinician Scientist Academy (UMEA) sponsored by the faculty of medicine and Deutsche Forschungsgemeinschaft (DFG). The rest of the authors (IGM, LSA, AKCT, FG, and RCDB) report they have no conflict of interest. Research involving human participants and/or animals: This study is a review. Therefore, this is not applicable. Informed consent: This study is a review. Therefore, this is not applicable.
Figures


References
-
- Jadvar H, Colletti PM, Delgado-Bolton R, Esposito G, Krause BJ, Iagaru AH, et al. Appropriate use criteria for 18F-FDG PET/CT in restaging and treatment response assessment of malignant disease. J Nucl Med. 2017;58:2026–37. 10.2967/jnumed.117.197988. - PubMed
-
- Hicks RJ, Roselt PJ, Kallur KG, Tothill RW, Mileshkin L. FAPI PET/CT: Will It End the Hegemony of 18F-FDG in Oncology? J Nucl Med. 2021;62:296–302. 10.2967/jnumed.120.256271. - PubMed
-
- Mori Y, Dendl K, Cardinale J, Kratochwil C, Giesel FL, Haberkorn U. FAPI PET: fibroblast activation protein inhibitor use in oncologic and nononcologic disease. Radiology. 2023;306: e220749. 10.1148/radiol.220749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

