Efficacy and continuability of 675 mg fremanezumab administration over 2 years
- PMID: 40069630
- PMCID: PMC11898999
- DOI: 10.1186/s10194-025-01994-5
Efficacy and continuability of 675 mg fremanezumab administration over 2 years
Abstract
Background: Real-world data on the long-term adherence to- and efficacy of fremanezumab 675 mg quarterly dosing remain scarce. Our study evaluated the efficacy of- and patient adherence to 675 mg fremanezumab for episodic migraine (EM) and chronic migraine (CM) over 2 years and analyzed the reasons for discontinuation.
Methods: Among patients attending our headache outpatient clinic, those aged ≥ 15 years who commenced fremanezumab 675 mg quarterly dose schedule from November 2021 to June 2022 were enrolled in this single-center observational study. The frequency and severity of headaches were recorded using a headache diary. The observation period ended for each patient at 24 months after treatment initiation. The reasons for discontinuation were documented based on follow-up medical records.
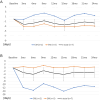
Results: Twenty-eight patients were enrolled, of whom 15 had CM and 13 had EM. One patient with CM was excluded due to withdrawal after the first injection. Of the 27 remaining patients, the treatment was effective in 70.4% (n = 19). 44.4% (n = 12) continued fremanezumab 675 mg until study termination. Among those patients who remained on fremanezumab for two years, seven updated the monthly headache calendars consistently: 2 had CM, and 5 had EM. Mean changes in MMD from the baseline were - 2.2 at 3 months,, -1.8 at 12 months, and - 1.6 (SD = 3.0) at 2 years. Treatment was discontinued because of sustained improvement in 25.9% (n = 7). 22.2% of cases (n = 6) experienced insufficient effectiveness, resulting in discontinuation. One patient (3.7%) discontinued because of injection-site erythema. One patient (3.7%) was discontinued because of pregnancy. Among the non-responders, three switched from fremanezumab to erenumab, with one returning to fremanezumab at a monthly injection of 225 mg after efficacy with erenumab waned. Two patients switched to galcanezumab. All patients who switched medication continued the new medication owing to its effectiveness. One patient was lost to follow-up.
Conclusions: Fremanezumab 675 mg quarterly dose effectively reduces headache frequency over an extended period and may facilitate medication cessation in patients who experience substantial recovery.
Keywords: Adherence; Calcitonin gene-related peptide; Migraine; Monoclonal antibodies; Real-world data.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by Ethics Committee of Japanese Red Cross Society Shizuoka Hospital (approval number: 2018-21). Patients were informed about this observational study via the questionnaire of the headache outpatient clinic, and they could opt out of this study. The need for informed consent was waived by Japanese Red Cross Society Shizuoka hospital in accordance with national regulations (Ethical Guidelines for Medical and Biological Research Involving Human Subjects). All methods were carried out in accordance with relevant guidelines and regulations. Consent for publication: Not applicable for that section. Competing interests: Noboru Imai reports being an advisor for Sawai and received speaker fees from Daiichi Sankyo, Eli Lilly, Otsuka, and Amgen; however, these companies had no relation to the study.
Figures

References
-
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia (2018);38(1):1-211. 10.1177/0333102417738202. PMID: 29368949 - PubMed
-
- Lipton RB, Cohen JM, Galic M, Seminerio MJ, Yeung PP, Aycardi E, Bigal ME, Bibeau K, Buse DC (2021) Effects of fremanezumab in patients with chronic migraine and comorbid depression: subgroup analysis of the randomized HALO CM study. Headache 61(4):662–672. 10.1111/head.14097PMID: 33891348; PMCID: PMC8251795 - PMC - PubMed
-
- Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, Ashina M, van den Maagdenberg AMJM, Dodick DW, Migraine (2022) Nat Rev Dis Primers.;8(1):2. 10.1038/s41572-021-00328-4. PMID: 35027572 - PubMed
-
- Peres MFP, Sacco S, Pozo-Rosich P, Tassorelli C, Ahmed F, Burstein R, Ashina S, Uluduz D, Husøy AK, Steiner TJ (2024) Migraine is the most disabling neurological disease among children and adolescents, and second after stroke among adults: A call to action. Cephalalgia.;44(8):3331024241267309. 10.1177/03331024241267309. PMID: 39197864 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

