Dys-regulated phosphatidylserine externalization as a cell intrinsic immune escape mechanism in cancer
- PMID: 40069722
- PMCID: PMC11900106
- DOI: 10.1186/s12964-025-02090-6
Dys-regulated phosphatidylserine externalization as a cell intrinsic immune escape mechanism in cancer
Abstract
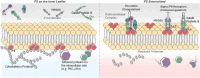
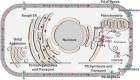
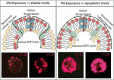
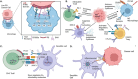
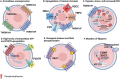
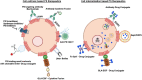
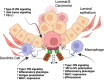
The negatively charged aminophospholipid, phosphatidylserine (PS), is typically restricted to the inner leaflet of the plasma membrane under normal, healthy physiological conditions. PS is irreversibly externalized during apoptosis, where it serves as a signal for elimination by efferocytosis. PS is also reversibly and transiently externalized during cell activation such as platelet and immune cell activation. These events associated with physiological PS externalization are tightly controlled by the regulated activation of flippases and scramblases. Indeed, improper regulation of PS externalization results in thrombotic diseases such as Scott Syndrome, a defect in coagulation and thrombin production, and in the case of efferocytosis, can result in autoimmunity such as systemic lupus erythematosus (SLE) when PS-mediated apoptosis and efferocytosis fails. The physiological regulation of PS is also perturbed in cancer and during viral infection, whereby PS becomes persistently exposed on the surface of such stressed and diseased cells, which can lead to chronic thrombosis and chronic immune evasion. In this review, we summarize evidence for the dysregulation of PS with a main focus on cancer biology and the pathogenic mechanisms for immune evasion and signaling by PS, as well as the discussion of new therapeutic strategies aimed to target externalized PS. We posit that chronic PS externalization is a universal and agnostic marker for diseased tissues, and in cancer, likely reflects a cell intrinsic form of immune escape. The continued development of new therapeutic strategies for targeting PS also provides rationale for their co-utility as adjuvants and with immune checkpoint therapeutics.
Keywords: Immune escape; P4 ATPase; PS receptors; Phosphatidylserine; Scramblases.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: SVK and RBB are co-founders of a biotech company called Targeron Therapeutics that aim to develop PS-targeting biologicals. Other authors have nothing to declare.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

