Young fibroblast-derived migrasomes alleviate keratinocyte senescence and enhance wound healing in aged skin
- PMID: 40069826
- PMCID: PMC11895310
- DOI: 10.1186/s12951-025-03293-2
Young fibroblast-derived migrasomes alleviate keratinocyte senescence and enhance wound healing in aged skin
Abstract
Background: Alterations in intercellular communication driven by cellular senescence constitute an important factor in skin aging. Migrasome, a newly discovered vesicular organelle, efficiently participates in intercellular communication; however, the relationship between cellular senescence and migrasomes remains unreported.
Objective: This study aims to explore the possible relationship between cellular senescence and migrasomes formation, and investigate the effects of young fibroblast-derived migrasomes on senescent keratinocytes and wound healing in aged skin.
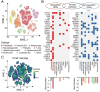
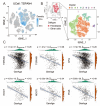
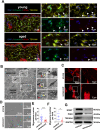
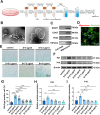
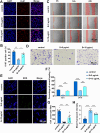
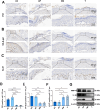
Result: Single-cell RNA sequencing (scRNA-seq) data analysis revealed that fibroblasts exhibited the highest level of transcriptional variability during skin aging, and the degree of fibroblast senescence negatively correlated with the expression level of migrasome-associated markers. Further multiplex Immunohistochemistry (mIHC) results suggested that younger mouse skin contained more migrasomes than older mouse skin. Transmission electron microscopy (TEM) observations demonstrated abundant migrasomes in the skin from young individuals. In vitro experiments indicated that young fibroblasts produced significantly more migrasomes than senescent fibroblasts, as confirmed by wheat germ agglutinin (WGA) staining and scanning electron microscopy (SEM). Importantly, purified migrasomes from young fibroblasts were found to reduce the expression of senescence-associated markers in HaCaT cells. In vivo, using a wound healing model in naturally aged mice, we observed that migrasomes derived from young fibroblasts not only accelerated wound healing but also reduced senescence-associated marker expression in the skin.
Conclusion: Migrasomes formation ability reduced during skin aging progress, and young fibroblast-derived migrasomes rejuvenated senescent keratinocytes and promoted wound healing in aged skin. These findings offer new ideas for alleviating skin aging and enhancing wound healing in aged skin.
Keywords: Aging; Fibroblast; Migrasomes; Senescence; Skin wound healing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Single-Cell Analysis Reveals Fibroblast-Derived Migrasomes as CXCL12 Carriers Promoting Skin Wound Repair.J Extracell Vesicles. 2025 Jun;14(6):e70112. doi: 10.1002/jev2.70112. J Extracell Vesicles. 2025. PMID: 40527746 Free PMC article.
-
Sustained release of migrasomes from a methacrylate-oxidized hyaluronic acid/methacrylated gelatin composite hydrogel accelerates skin wound healing.Int J Biol Macromol. 2025 May;306(Pt 1):141355. doi: 10.1016/j.ijbiomac.2025.141355. Epub 2025 Feb 21. Int J Biol Macromol. 2025. PMID: 39988172
-
Induction of Fibroblast Senescence During Mouse Corneal Wound Healing.Invest Ophthalmol Vis Sci. 2019 Aug 1;60(10):3669-3679. doi: 10.1167/iovs.19-26983. Invest Ophthalmol Vis Sci. 2019. PMID: 31469894
-
Cell Autonomous and Non-Autonomous Effects of Senescent Cells in the Skin.J Invest Dermatol. 2015 Jul;135(7):1722-1726. doi: 10.1038/jid.2015.108. Epub 2015 Apr 9. J Invest Dermatol. 2015. PMID: 25855157 Free PMC article. Review.
-
Cell aging and cellular senescence in skin aging - Recent advances in fibroblast and keratinocyte biology.Exp Gerontol. 2020 Feb;130:110780. doi: 10.1016/j.exger.2019.110780. Epub 2019 Nov 30. Exp Gerontol. 2020. PMID: 31794850 Review.
References
-
- Velarde MC, Demaria M. Targeting senescent cells: possible implications for delaying skin aging: A Mini-Review. Gerontology. 2016;62:513–8. - PubMed
-
- Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: a mini-review. Gerontology. 2013;59:159–64. - PubMed
-
- Wang B, Han J, Elisseeff JH, Demaria M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat Rev Mol Cell Biol. 2024;25:958–78. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

