Enhanced mitochondrial function and delivery from adipose-derived stem cell spheres via the EZH2-H3K27me3-PPARγ pathway for advanced therapy
- PMID: 40069892
- PMCID: PMC11899936
- DOI: 10.1186/s13287-025-04164-1
Enhanced mitochondrial function and delivery from adipose-derived stem cell spheres via the EZH2-H3K27me3-PPARγ pathway for advanced therapy
Abstract
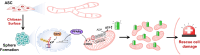
Background: Microenvironmental alterations induce significant genetic and epigenetic changes in stem cells. Mitochondria, essential for regenerative capabilities, provide the necessary energy for stem cell function. However, the specific roles of histone modifications and mitochondrial dynamics in human adipose-derived stem cells (ASCs) during morphological transformations remain poorly understood. In this study, we aim to elucidate the mechanisms by which ASC sphere formation enhances mitochondrial function, delivery, and rescue efficiency.
Methods: ASCs were cultured on chitosan nano-deposited surfaces to form 3D spheres. Mitochondrial activity and ATP production were assessed using MitoTracker staining, Seahorse XF analysis, and ATP luminescence assays. Single-cell RNA sequencing, followed by Ingenuity Pathway Analysis (IPA), was conducted to uncover key regulatory pathways, which were validated through molecular techniques. Pathway involvement was confirmed using epigenetic inhibitors or PPARγ-modulating drugs. Mitochondrial structural integrity and delivery efficiency were evaluated after isolation.
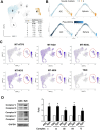
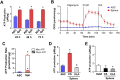
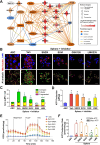
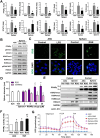
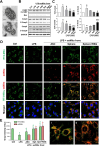
Results: Chitosan-induced ASC spheres exhibited unique compact mitochondrial morphology, characterized by condensed cristae, enhanced mitochondrial activity, and increased ATP production through oxidative phosphorylation. High expressions of mitochondrial complex I genes and elevated levels of mitochondrial complex proteins were observed without an increase in reactive oxygen species (ROS). Epigenetic modification of H3K27me3 and PPARγ involvement were discovered and confirmed by inhibiting H3K27me3 with the specific EZH2 inhibitor GSK126 and by adding the PPARγ agonist Rosiglitazone (RSG). Isolated mitochondria from ASC spheres showed improved structural stability and delivery efficiency, suppressed the of inflammatory cytokines in LPS- and TNFα-induced inflamed cells, and rescued cells from damage, thereby enhancing function and promoting recovery.
Conclusion: Enhancing mitochondrial ATP production via the EZH2-H3K27me3-PPARγ pathway offers an alternative strategy to conventional cell-based therapies. High-functional mitochondria and delivery efficiency show significant potential for regenerative medicine applications.
Keywords: 3D spheroid culture; Adipose-derived stem cells; Chitosan nano-deposition; EZH2-H3K27me3-PPARγ pathway; Enhanced mitochondrial function; Mitochondrial therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This article does not include any studies involving human participants conducted by any of the authors. Human ASCs (C12977, hMSC-AT) used in this research were purchased from PromoCell GmbH. Detailed information regarding compliance and prior ethical approval can be found on the company’s official website: PromoCell Compliance. The Hs68 cell line (BCRC No. 60038) was obtained from BCRC of FIRDI, Hsinchu, Taiwan. The Material Transfer Agreement (MTA) and product certificates are included in Supplementary Material 13. Consent for publication: Not applicable. Competing interests: The authors declare no conflict of interest.
Figures

References
-
- Vogt N, Assembloids. Nat Methods. 2021;18(1):27. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

