The Combination of PARP and Topoisomerase 1 Inhibitors Improves Radiation Therapy for Ewing Sarcoma
- PMID: 40069935
- PMCID: PMC12127087
- DOI: 10.1111/cas.70042
The Combination of PARP and Topoisomerase 1 Inhibitors Improves Radiation Therapy for Ewing Sarcoma
Abstract
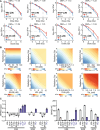
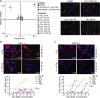
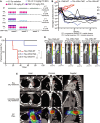
Although primary tumor control rates after surgery and/or radiation therapy (RT) are generally high in patients with Ewing sarcoma (EWS), those with unresectable tumors have failure rates approaching 30% and experience poorer outcomes. Additionally, although metastatic site irradiation is associated with improved survival, dose, and volume effects influence the long-term toxicity risk. Consequently, it is important to identify novel systemic agents to enhance the therapeutic ratio of RT. Given the reported DNA damage response deficits in EWS, we hypothesized that PARP inhibitors (PARPis) would preferentially potentiate radiation relative to standard-of-care (SOC) chemotherapeutics. We investigated primary and recurrent SOC drugs and PARPis with varied trapping potential in combination with radiation in EWS cell lines. At physiologically relevant concentrations, the strong PARP trapper talazoparib (TAL) potentiated radiation to a greater extent than did SOC or other PARPis, although the magnitude of the effect was modest. The radiosensitizing effect of TAL was mediated through the induction of DNA double-strand breaks, rather than through the catalytic inhibition of PARP1. Drug + RT combinations were further tested in vivo by using orthotopic xenograft models of EWS treated with image-guided fractionated radiation. The addition of RT to the combination of TAL plus irinotecan (IRN), a recently evaluated clinical regimen for relapsed pediatric solid tumors, significantly prolonged survival and reduced tumor burden in all EWS-treated mice. This triplet therapy (TAL + IRN + RT) was feasible and yielded responses in several patients with EWS and may represent a useful salvage strategy in recurrent or progressive disease.
Keywords: DNA damage; Ewing sarcoma; PARP inhibitors; radiation therapy; topoisomerase I inhibitors.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have nothing to report.
The authors declare no conflicts of interest.
Figures


References
-
- Ewing J., “Classics in Oncology. Diffuse Endothelioma of Bone. James Ewing. Proceedings of the New York Pathological Society, 1921,” CA: A Cancer Journal for Clinicians 22, no. 2 (1972): 95–98. - PubMed
-
- Arai Y., Kun L. E., Brooks M. T., et al., “Ewing's Sarcoma: Local Tumor Control and Patterns of Failure Following Limited‐Volume Radiation Therapy,” International Journal of Radiation Oncology, Biology, Physics 21, no. 6 (1991): 1501–1508. - PubMed
-
- Rodriguez‐Galindo C., Navid F., Liu T., Billups C. A., Rao B. N., and Krasin M. J., “Prognostic Factors for Local and Distant Control in Ewing Sarcoma Family of Tumors,” Annals of Oncology 19, no. 4 (2008): 814–820. - PubMed
-
- Talleur A. C., Navid F., Spunt S. L., et al., “Limited Margin Radiation Therapy for Children and Young Adults With Ewing Sarcoma Achieves High Rates of Local Tumor Control,” International Journal of Radiation Oncology, Biology, Physics 96, no. 1 (2016): 119–126, 10.1016/j.ijrobp.2016.04.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

