Computational tools for the prediction of site- and regioselectivity of organic reactions
- PMID: 40070469
- PMCID: PMC11891785
- DOI: 10.1039/d5sc00541h
Computational tools for the prediction of site- and regioselectivity of organic reactions
Abstract
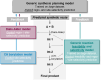
The regio- and site-selectivity of organic reactions is one of the most important aspects when it comes to synthesis planning. Due to that, massive research efforts were invested into computational models for regio- and site-selectivity prediction, and the introduction of machine learning to the chemical sciences within the past decade has added a whole new dimension to these endeavors. This review article walks through the currently available predictive tools for regio- and site-selectivity with a particular focus on machine learning models while being organized along the individual reaction classes of organic chemistry. Respective featurization techniques and model architectures are described and compared to each other; applications of the tools to critical real-world examples are highlighted. This paper aims to serve as an overview of the field's status quo for both the intended users of the tools, that is synthetic chemists, as well as for developers to find potential new research avenues.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




















Similar articles
-
ASKCOS: Open-Source, Data-Driven Synthesis Planning.Acc Chem Res. 2025 Jun 3;58(11):1764-1775. doi: 10.1021/acs.accounts.5c00155. Epub 2025 May 21. Acc Chem Res. 2025. PMID: 40397546
-
Importance of Engineered and Learned Molecular Representations in Predicting Organic Reactivity, Selectivity, and Chemical Properties.Acc Chem Res. 2021 Feb 16;54(4):827-836. doi: 10.1021/acs.accounts.0c00745. Epub 2021 Feb 3. Acc Chem Res. 2021. PMID: 33534534
-
Machine Learning in Computer-Aided Synthesis Planning.Acc Chem Res. 2018 May 15;51(5):1281-1289. doi: 10.1021/acs.accounts.8b00087. Epub 2018 May 1. Acc Chem Res. 2018. PMID: 29715002
-
Bridging Chemical Knowledge and Machine Learning for Performance Prediction of Organic Synthesis.Chemistry. 2023 Jan 27;29(6):e202202834. doi: 10.1002/chem.202202834. Epub 2022 Nov 27. Chemistry. 2023. PMID: 36206170 Free PMC article. Review.
-
Recent applications of machine learning in medicinal chemistry.Bioorg Med Chem Lett. 2018 Sep 15;28(17):2807-2815. doi: 10.1016/j.bmcl.2018.06.046. Epub 2018 Jun 28. Bioorg Med Chem Lett. 2018. PMID: 30122222 Review.
References
-
- Biyani S. A. Moriuchi Y. W. Thompson D. H. Chem. Methods. 2021;1:323–339. doi: 10.1002/cmtd.202100023. - DOI
-
- Berritt S., Christensen M., Johansson M. J., Krska S. W., Newman S. G., Sampson J., Simmons E. M., Wang Y. and Strotman N. A., in The Power of High-Throughput Experimentation: General Topics and Enabling Technologies for Synthesis and Catalysis (Volume 1), American Chemical Society, 2022, vol. 1419, ch. 1, pp. 3–9
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

