Development of an enzyme-linked immunosorbent assay (ELISA) for determining neutrophil elastase (NE) - a potential useful marker of multi-organ damage observed in COVID-19 and post-Covid-19 (PCS)
- PMID: 40070691
- PMCID: PMC11893405
- DOI: 10.3389/fmolb.2025.1542898
Development of an enzyme-linked immunosorbent assay (ELISA) for determining neutrophil elastase (NE) - a potential useful marker of multi-organ damage observed in COVID-19 and post-Covid-19 (PCS)
Abstract
Background: The ongoing post-COVID-19 syndrome (PCS) epidemic, causing complications of diverse etiology, necessitates the search for new diagnostic markers and the development of widely accessible methods for their detection. This would enable the prognosis of PCS progression and faster implementation of targeted treatments. One potential marker is neutrophil elastase (NE), whose elevated levels in the blood during PCS may result from organ damage caused by increased secretion of severe inflammatory mediators or amyloidosis resulting from the interaction of NE with SARS-CoV-2. The aim of this publication is to present a step-by-step method for designing an enzymatic ELISA test, enabling the quantitative assessment of NE in the blood serum of patients.
Methods: NE was measured using the designed ELISA test.
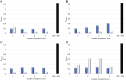
Results: The study outlines all the steps necessary for designing and optimizing the ELISA test, including the selection of standards, primary and secondary antibodies, and their dilutions. Using the test, elevated NE levels were demonstrated in patients with advanced-stage diabetic nephropathy after symptomatic COVID-19, compared to a relative group of patients sampled before COVID-19.
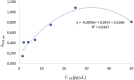
Conclusion: The undertaken efforts enabled the development of a test with high performance parameters (initially set sensitivity: ≥40 pg/μL; intra-assay precision: 7%; inter-assay precision <20%). No significant cross-reactivity with other tested proteins was observed. Serial dilution of plasma samples resulted in a proportional decrease in signal intensity.
Keywords: COVID-19; ELISA; long-COVID biomarkers; neutrophil elastase 1; post-COVID-19-syndrome (PCS).
Copyright © 2025 Adamiec-Mroczek, Kluz, Chwałek, Rabczyński, Gostomska-Pampuch, Lewandowski, Misiuk-Hojło, Ponikowska, Chourasia, Dumas, Gamian, Fiodorenko-Dumas, Konopska, Gola, Konikowska, Strub, Bronowicka-Szydełko and Madziarska.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











References
-
- Allam A. A. E. H., Eltaras S. M., Hussin M. H., Salama E. S. I., Hendy O. M., Allam M. M., et al. (2018). Diagnosis of spontaneous bacterial peritonitis in children using leukocyte esterase reagent strips and granulocyte elastase immunoassay. Clin. Exp. Hepatol. 4, 247–252. 10.5114/CEH.2018.80126 - DOI - PMC - PubMed
-
- Aruta M. G., Lari E., De Simone D., Semplici B., Semplici C., Dale H., et al. (2023). Characterization of enzyme-linked immunosorbent assay (ELISA) for quantification of antibodies against Salmonella typhimurium and Salmonella enteritidis O-antigens in human sera, BioTech. 12. 54. 10.3390/BIOTECH12030054 - DOI - PMC - PubMed
-
- Bronowicka-Szydełko A., Krzystek-Korpacka M., Gacka M., Pietkiewicz J., Jakobsche-Policht U., Gamian A. (2021). Association of novel advanced glycation end-product (AGE10) with complications of diabetes as measured by enzyme-linked immunosorbent assay. J. Clin. Med. 10, 4499. 10.3390/JCM10194499 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

