Human pluripotent stem cell-derived hepatic progenitors exhibit a partially hypoimmunogenic phenotype and actively inhibit immune responses
- PMID: 40070824
- PMCID: PMC11893836
- DOI: 10.3389/fimmu.2025.1507317
Human pluripotent stem cell-derived hepatic progenitors exhibit a partially hypoimmunogenic phenotype and actively inhibit immune responses
Abstract
Introduction: GStemHep cells are human cryopreserved hepatic progenitors derived from pluripotent of stem cells (GStem cells) using a cGMP-compliant protocol. They were highly effective in rescuing mice from acute liver failure.
Methods: The objective of this study was to analyze the immunogenicity and immunoregulatory properties of GStemHep cells.
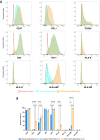
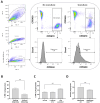
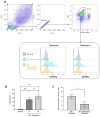
Results: As compared to GStem cells, GStemHep cells showed complete loss of HLA-I (ABC) and they lacked of expression of HLA-II, HLA-G, HLA-E and PD-L1. GStemHep cells also showed increased expression of CD47, maintained high expression of indoleamine 2,3-dioxygenase (IDO) and heme oxygenase-1 (HO-1) and reduced expression of CD200. In comparison with GStem cells, GStemHep cultured in inflammatory conditions increased the expression of PD-L1, CD200, HO-1, HLA-E, CD47 and HLA-I (ABC) as well as maintained expression of IDO and were negative for HLA-II and HLA-G. GStemHep culture in basal or inflammatory conditions has a low or absent immunogenic activity on T cells, associated to a suppressive effect on proliferation partially mediated by IDO. We observed phagocytosis of GStemHep by macrophages that was partially inhibited by CD47 expression. NK cells were activated by resting GStemHep cells. Upon culture in inflammatory conditions that induced expression of HLA-I molecules in GStemHep cells NK cell activation was reduced. Thus, GStemHep cells are partially hypoimmune cells due to the expression of several immune checkpoint inhibitors and the absence of HLA-I molecules. In inflammatory conditions, the expression of several of these molecules was increased but also of HLA-I that could be immunogenic for T cells but it was inhibitory for NK cells.
Discussion: GStemHep cells show a favorable immunological profile for their use as allogeneic off-the shelf treatment of liver diseases with loss of hepatocyte function.
Keywords: hepatic progenitors; liver; pluripotent stem cells; tolerance; transplantation.
Copyright © 2025 Gantier, Ménoret, Fourrier, Delbos, Nguyen and Anegon.
Conflict of interest statement
MG, AF, FD and TN are employees of Goliver Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fourrier A, Delbos F, Menoret S, Collet C, Thi Thuy LT, Myara A, et al. . Regenerative cell therapy for the treatment of hyperbilirubinemic Gunn rats with fresh and frozen human induced pluripotent stem cells-derived hepatic stem cells. Xenotransplantation. (2020) 27. doi: 10.1111/xen.12544 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

