Electroacupuncture Inhibits NLRP3-Mediated Microglial Pyroptosis to Ameliorate Chronic Neuropathic Pain in Rats
- PMID: 40070891
- PMCID: PMC11895692
- DOI: 10.2147/JPR.S506569
Electroacupuncture Inhibits NLRP3-Mediated Microglial Pyroptosis to Ameliorate Chronic Neuropathic Pain in Rats
Abstract
Background: Patients with neuropathic pain (NP), caused by injury or disease of the somatosensory nervous system, usually suffer from severe pain. Our previous studies revealed that electroacupuncture (EA) stimulation could effectively improve NP. However, the underlying mechanisms of EA have not been fully clarified. This study aimed to investigate the specific mechanisms of EA in alleviating NP, focusing on the pyroptosis.
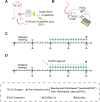
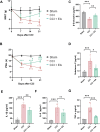
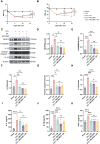
Materials and methods: Chronic Constriction Injury (CCI) model was established on the male Sprague-Dawley rats. CCI rats were treated with EA at acupoints GV20 and ST36 or/with the NOD-like receptor protein 3 (NLRP3) antagonist MCC950. EA treatment was administered for successive 14 days 7 days after the CCI surgery. The mechanical withdrawal threshold (MWT) and paw withdrawal latency (PWL) were performed during the experiment. At the end of the experiment, spinal cord segments and serum of rats were collected, ELISA detected the expression of inflammatory factors, immunofluorescence detected the microglia and neuron cells with pyroptosis biomarkers, and Western blot detected the NLRP3 pathway.
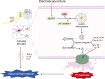
Results: EA treatment significantly alleviated pain hypersensitivity by increasing the MWT and PWL. Moreover, EA reduced levels of pro-inflammatory cytokines IL-1β and TNF-α in the spinal tissue. Mechanistically, the pyroptosis-related proteins, including NLRP3, N-GSDMD, Cleaved Caspase-1, IL-18 as well as IL-1β were downregulated by EA, indicating that EA attenuated the pyroptosis phenotype in NP rats. In particular, EA reduced the co-expression of NLRP3, Caspase-1 and N-GSDMD in microglia rather than in neuronal or astrocytic cells within the spinal cord of CCI rats. Pharmacological inhibition of NLRP3 inflammasome by MCC950 alleviates CCI-induced pain hypersensitivity while blocking EA's effect on anti-pyroptosis in CCI rats.
Conclusion: These findings demonstrate the EA ameliorates the neuroinflammation and pyroptosis to relieve chronic NP by suppressing NLRP3 inflammasome activation in microglia. EA may serve as a viable treatment therapy for chronic NP.
Keywords: NLRP3 inflammasome; electroacupuncture; neuroinflammation; neuropathic pain; pyroptosis.
© 2025 Kui et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

