IQGAP2 regulates blood-brain barrier immune dynamics
- PMID: 40071147
- PMCID: PMC11894336
- DOI: 10.1016/j.isci.2025.111994
IQGAP2 regulates blood-brain barrier immune dynamics
Abstract
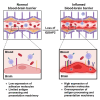
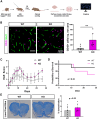
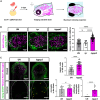
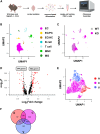
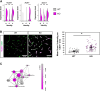
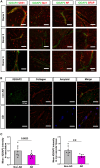
Brain endothelial cells (BECs) play an important role in maintaining central nervous system (CNS) homeostasis through blood-brain barrier (BBB) functions. BECs express low baseline levels of adhesion receptors, which limits entry of leukocytes. However, the molecular mediators governing this phenotype remain mostly unclear. Here, we explored how infiltration of immune cells across the BBB is influenced by the scaffold protein IQ motif containing GTPase-activating protein 2 (IQGAP2). In mice and zebrafish, we demonstrate that loss of Iqgap2 increases infiltration of peripheral leukocytes into the CNS under homeostatic and inflammatory conditions. Using single-cell RNA sequencing and immunohistology, we further show that BECs from mice lacking Iqgap2 exhibit a profound inflammatory signature, including extensive upregulation of adhesion receptors and antigen-processing machinery. Human tissue analyses also reveal that Alzheimer's disease is associated with reduced hippocampal IQGAP2. Overall, our results implicate IQGAP2 as an essential regulator of BBB immune privilege and immune cell entry into the CNS.
Keywords: immunology; neuroscience.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Update of
-
IQGAP2 regulates blood-brain barrier immune dynamics.bioRxiv [Preprint]. 2024 Apr 12:2023.02.07.527394. doi: 10.1101/2023.02.07.527394. bioRxiv. 2024. Update in: iScience. 2025 Feb 11;28(3):111994. doi: 10.1016/j.isci.2025.111994. PMID: 38645082 Free PMC article. Updated. Preprint.
Similar articles
-
IQGAP2 regulates blood-brain barrier immune dynamics.bioRxiv [Preprint]. 2024 Apr 12:2023.02.07.527394. doi: 10.1101/2023.02.07.527394. bioRxiv. 2024. Update in: iScience. 2025 Feb 11;28(3):111994. doi: 10.1016/j.isci.2025.111994. PMID: 38645082 Free PMC article. Updated. Preprint.
-
IQ Motif-Containing GTPase-Activating Protein 2 (IQGAP2) Is a Novel Regulator of Colonic Inflammation in Mice.PLoS One. 2015 Jun 5;10(6):e0129314. doi: 10.1371/journal.pone.0129314. eCollection 2015. PLoS One. 2015. PMID: 26047140 Free PMC article.
-
Depletion of IQ motif-containing GTPase activating protein 2 (IQGAP2) reduces hepatic glycogen and impairs insulin signaling.J Biol Chem. 2023 Nov;299(11):105322. doi: 10.1016/j.jbc.2023.105322. Epub 2023 Oct 5. J Biol Chem. 2023. PMID: 37805137 Free PMC article.
-
Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review.Histol Histopathol. 2004 Apr;19(2):535-64. doi: 10.14670/HH-19.535. Histol Histopathol. 2004. PMID: 15024715 Review.
-
The blood-brain barrier and its role in immune privilege in the central nervous system.J Neuropathol Exp Neurol. 2003 Jun;62(6):593-604. doi: 10.1093/jnen/62.6.593. J Neuropathol Exp Neurol. 2003. PMID: 12834104 Review.
Cited by
-
Decoding neuroinflammation in Alzheimer's disease: a multi-omics and AI-driven perspective for precision medicine.Front Immunol. 2025 Jun 18;16:1616899. doi: 10.3389/fimmu.2025.1616899. eCollection 2025. Front Immunol. 2025. PMID: 40607399 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

