An NRF2/β3-Adrenoreceptor Axis Drives a Sustained Antioxidant and Metabolic Rewiring Through the Pentose-Phosphate Pathway to Alleviate Cardiac Stress
- PMID: 40071326
- PMCID: PMC12052078
- DOI: 10.1161/CIRCULATIONAHA.124.067876
An NRF2/β3-Adrenoreceptor Axis Drives a Sustained Antioxidant and Metabolic Rewiring Through the Pentose-Phosphate Pathway to Alleviate Cardiac Stress
Abstract
Background: Cardiac β3-adrenergic receptors (ARs) are upregulated in diseased hearts and mediate antithetic effects to those of β1AR and β2AR. β3AR agonists were recently shown to protect against myocardial remodeling in preclinical studies and to improve systolic function in patients with severe heart failure. However, the underlying mechanisms remain elusive.
Methods: To dissect functional, transcriptional, and metabolic effects, hearts and isolated ventricular myocytes from mice harboring a moderate, cardiac-specific expression of a human ADRB3 transgene (β3AR-Tg) and subjected to transverse aortic constriction were assessed with echocardiography, RNA sequencing, positron emission tomography scan, metabolomics, and metabolic flux analysis. Subsequently, signaling and metabolic pathways were further investigated in vivo in β3AR-Tg and ex vivo in neonatal rat ventricular myocytes adenovirally infected to express β3AR and subjected to neurohormonal stress. These results were complemented with an analysis of single-nucleus RNA-sequencing data from human cardiac myocytes from patients with heart failure.
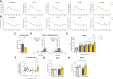
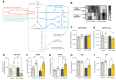
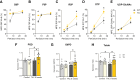
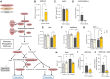
Results: Compared with wild-type littermates, β3AR-Tg mice were protected from hypertrophy after transaortic constriction, and systolic function was preserved. β3AR-expressing hearts displayed enhanced myocardial glucose uptake under stress in the absence of increased lactate levels. Instead, metabolomic and metabolic flux analyses in stressed hearts revealed an increase in intermediates of the pentose-phosphate pathway in β3AR-Tg, an alternative route of glucose utilization, paralleled with increased transcript levels of NADPH-producing and rate-limiting enzymes of the pentose-phosphate pathway, without fueling the hexosamine metabolism. The ensuing increased content of NADPH and of reduced glutathione decreased myocyte oxidant stress, whereas downstream oxidative metabolism assessed by oxygen consumption was preserved with higher glucose oxidation in β3AR-Tg mice after transaortic constriction compared with wild type, together with increased mitochondrial biogenesis. Unbiased transcriptomics and pathway analysis identified NRF2 (NFE2L2) as an upstream transcription factor that was functionally verified in vivo and in β3AR-expressing cardiac myocytes, where its translocation and nuclear activity were dependent on β3AR activation of nitric oxide synthase and nitric oxide production through S-nitrosation of the NRF2-negative regulator Keap1.
Conclusions: Moderate expression of cardiac β3AR, at levels observed in human cardiac myocardium, exerts metabolic and antioxidant effects through activation of the pentose-phosphate pathway and NRF2 pathway through S-nitrosation of Keap1, thereby preserving myocardial oxidative metabolism, function, and integrity under pathophysiological stress.
Keywords: hypertrophy; metabolism; myocytes, cardiac; oxidants; pentose phosphate pathway; receptors, adrenergic, beta-3.
Conflict of interest statement
None.
Figures



References
-
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB - PubMed
-
- Knoll R, Iaccarino G, Tarone G, Hilfiker-Kleiner D, Bauersachs J, Leite-Moreira AF, Sugden PH, Balligand JL; European Society of Cardiology. Towards a re-definition of “cardiac hypertrophy” through a rational characterization of left ventricular phenotypes: a position paper of the Working Group “Myocardial Function” of the ESC. Eur J Heart Fail. 2011;13:811–819. doi: 10.1093/eurjhf/hfr071 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

