Parvalbumin neurons mediate neurological phenotypes of anti-NMDAR encephalitis
- PMID: 40071389
- PMCID: PMC12073974
- DOI: 10.1093/brain/awae374
Parvalbumin neurons mediate neurological phenotypes of anti-NMDAR encephalitis
Abstract
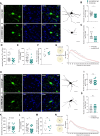
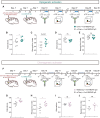
Patients with anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis, often present with severe psychiatric symptoms, yet the neuropathological mechanisms underlying their cognitive deficits remain insufficiently understood. In this study, we constructed an animal model using anti-NMDAR IgG purified from the serum of patients with anti-NMDAR encephalitis, and we used IgG obtained from healthy individuals as a control. Daily administration of anti-NMDAR IgG into the medial prefrontal cortex (mPFC) of mice for 7 days resulted in cognitive impairments resembling clinical symptoms, which spontaneously resolved 30 days after discontinuing the injections. Immunohistochemical staining and electrophysiological testing of parvalbumin neurons in the mPFC treated with anti-NMDAR IgG revealed significant cellular morphological damage, reduced excitability, synaptic dysfunction and a loss of NMDAR antagonist-induced gamma oscillations. Application of optogenetic and pharmacogenetic techniques to activate parvalbumin neurons in the mPFC successfully reversed the cognitive impairments observed in the anti-NMDAR-IgG-treated mice. Single-cell sequencing of anti-NMDAR-IgG-treated parvalbumin neurons identified differentially expressed genes and pathways related to synapses and neuronal development, offering potential targets for therapeutic intervention. Additionally, we showed that these alterations in parvalbumin neurons were not confined to the mPFC, as similar changes were detected in the hippocampus after anti-NMDAR IgG injections. In summary, our findings elucidate distinct alterations in parvalbumin neurons during the pathogenesis of anti-NMDAR encephalitis, providing preclinical rationale for exploring approaches to modulate parvalbumin neuronal function to treat anti-NMDAR encephalitis.
Keywords: NMDAR; cognitive impairments; gamma oscillations; medial prefrontal cortex; parvalbumin neurons; rescue.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Dalmau J, Armangué T, Planagumà J, et al. . An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: Mechanisms and models. Lancet Neurol. 2019;18:1045–1057. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

