Electrochemical Duplex Detection of E2 and E6 Genes of Human Papillomavirus Type 16 and Determination of Physical Status in High-Risk Cervical Carcinoma
- PMID: 40071579
- PMCID: PMC11898155
- DOI: 10.1002/jmv.70299
Electrochemical Duplex Detection of E2 and E6 Genes of Human Papillomavirus Type 16 and Determination of Physical Status in High-Risk Cervical Carcinoma
Abstract
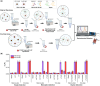
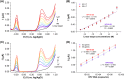
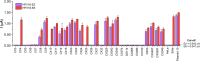
Human papillomavirus type 16 (HPV-16) is a key driver in the development of cervical carcinoma, with the integration of its genome into the host DNA marking a critical step in disease progression. Monitoring the physical state of HPV-16, particularly the transition from episomal to integrated forms, is essential for evaluating the risk of malignancy development in cervix. This study presents the development of a duplex electrochemical biosensor for the simultaneous detection of the E2 and E6 genes of HPV-16. Using a one-step sandwich hybridization assay, the biosensor was able to detect HPV-16 E2 and E6 genes with a sensitivity of 8 copies/mL and 12 copies/mL respectively and distinguish between the episomal and integrated forms based on the E2/E6 ratio (cut-off 0.77, 100% sensitivity/specificity). The sensor was validated with 30 clinical cervical tissue samples, providing results comparable to qPCR method. This novel biosensor offers a rapid and efficient platform for the detection and monitoring of HPV-16, with potential applications in cervical cancer screening and prognosis.
Keywords: cervical carcinoma; duplex detection; electrochemical sensor; human papillomavirus type 16; physical state.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
[Detection of physical status of human papillomavirus 16 in cervical cancer tissue and SiHa cell line by multiplex real-time polymerase chain reaction].Ai Zheng. 2006 Mar;25(3):373-7. Ai Zheng. 2006. PMID: 16536998 Chinese.
-
Viral DNA load, physical status and E2/E6 ratio as markers to grade HPV16 positive women for high-grade cervical lesions.Gynecol Oncol. 2007 Sep;106(3):549-57. doi: 10.1016/j.ygyno.2007.05.004. Epub 2007 Jun 13. Gynecol Oncol. 2007. PMID: 17568661
-
Human papillomavirus 16 E6/E7 transcript and E2 gene status in patients with cervical neoplasia.Mol Diagn. 2004;8(1):57-64. doi: 10.1007/BF03260048. Mol Diagn. 2004. PMID: 15230643
-
Cervical human papillomavirus screening by PCR: advantages of targeting the E6/E7 region.Clin Chem Lab Med. 2005;43(11):1171-7. doi: 10.1515/CCLM.2005.203. Clin Chem Lab Med. 2005. PMID: 16232081 Review.
-
Recent Advances in HPV Detection: From Traditional Methods to Nanotechnology and the Application of Quantum Dots.Int J Nanomedicine. 2025 May 21;20:6333-6356. doi: 10.2147/IJN.S524518. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40420910 Free PMC article. Review.
References
-
- Yadav G., Srinivasan G., and Jain A., “Cervical Cancer: Novel Treatment Strategies Offer Renewed Optimism,” Pathology ‐ Research and Practice 254 (2024): 155136. - PubMed
-
- Bray F., Laversanne M., Sung H., et al., “Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 74 (2024): 229–263. - PubMed
-
- Ong S. K., Abe S. K., Thilagaratnam S., et al., “Towards Elimination of Cervical Cancer ‐ Human Papillomavirus (HPV) Vaccination and Cervical Cancer Screening in Asian National Cancer Centers Alliance (ANCCA) Member Countries,” Lancet Regional Health ‐ Western Pacific 39 (2023): 100860. - PMC - PubMed

