Patients' experiences with GLP1-RAs - a systematic review
- PMID: 40071612
- PMCID: PMC12090293
- DOI: 10.1080/02813432.2025.2477141
Patients' experiences with GLP1-RAs - a systematic review
Abstract
Background: Obesity is a complex condition and a recognized public health challenge. Previous treatment options were associated with high failure rates, but recent trials have shown that significant weight loss can be achieved with GLP1-RAs. However, little is known about the patient's experiences with GLP1-RAs.
Objectives: This paper systematically reviews research on patients' experience with GLP1-RAs.
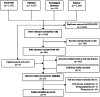
Methods: A literature search in PubMed, PsycINFO, Embase and Sociological Abstracts included studies on adults' experiences with GLP1-RAs, regardless of methodology. Exclusions of studies: mental illness, pregnancy, former bariatric surgery, PCOS. Study quality and transparency were assessed according to design, using thematic analysis for synthesis.
Results: Nine studies, selected from 7,607 records, encompassed three qualitative studies (semi-structured interviews), three RCTs, two narrative reviews and one survey study. The analysis identified five key themes: (1) Patients are willing to accept adverse events, like gastrointestinal disorders, for successful weight loss, (2) Patients experience improved physical functioning, well-being, and active daily living as a result of weight loss, (3) Patients express diverse opinions and skills regarding the medication's usability, (4) Patients believe that the medication improves their ability to manage sweet cravings, (5) Gender seems to affect patients' experiences with the medication, with females reporting more benefits than males.
Conclusion: Despite a huge demand and usage of GLP1-RAs, qualitative research on patients' experiences is scarce. Further studies are crucial for understanding short and long-term patient experiences.
Keywords: GLP1-RA; Lived experiences; perception; perspective; systematic review.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- Wadden TA, Bailey TS, Billings LK, et al. . Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413. doi: 10.1001/jama.2021.1831. - DOI - PMC - PubMed
-
- Collins L, Costello RA.. Glucagon-like peptide-1 receptor agonists. 2019. https://www.ncbi.nlm.nih.gov/books/NBK551568/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical