Distinct Network Morphologies from In Situ Polymerization of Microtubules in Giant Polymer-Lipid Hybrid Vesicles
- PMID: 40071650
- PMCID: PMC12078856
- DOI: 10.1002/adbi.202400601
Distinct Network Morphologies from In Situ Polymerization of Microtubules in Giant Polymer-Lipid Hybrid Vesicles
Abstract
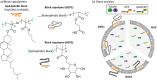
Creating artificial cells with a dynamic cytoskeleton, akin to those in living cells, is a major goal in bottom-up synthetic biology. In this study, we demonstrate the in situ polymerization of microtubules encapsulated in giant polymer-lipid hybrid vesicles (GHVs) composed of 1,2-dioleoyl-sn-glycero-3-phosphocholine and an amphiphilic block copolymer. The block copolymer is comprised of poly(cholesteryl methacrylate-co-butyl methacrylate) as the hydrophobic block and either poly(6-O-methacryloyl-D-galactopyranose) or poly(carboxyethyl acrylate) as the hydrophilic extension. Depending on the concentrations of guanosine triphosphate (GTP) or its slowly hydrolyzable analog, guanosine-5'-[(α,β)-methyleno]triphosphate (GMPCPP), different microtubule morphologies are observed, including encapsulated microtubule networks, spike protrusions, as well as membrane-associated or aggregated microtubules. Overall, this work represents a step forward in mimicking the cellular cytoskeletons and uncovering the influence of membrane composition on microtubule morphologies.
Keywords: artificial cells; giant hybrid vesicles; microtubules; tubulin polymerization.
© 2025 The Author(s). Advanced Biology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Chang M.‐Y., Ariyama H., Huck W. T. S., Deng N.‐N., Chem. Soc. Rev. 2023, 52, 3307. - PubMed
-
- a) Qian X., Westensee I. N., Fernandes C. C., Städler B., Angew. Chem. 2021, 133, 18852; - PubMed
- b) Westensee I. N., Städler B., Interface Focus 2023, 13, 20230007; - PMC - PubMed
- c) Shetty S. C., Yandrapalli N., Pinkwart K., Krafft D., Vidakovic‐Koch T., Ivanov I., Robinson T., ACS Nano 2021, 15, 15656; - PMC - PubMed
- d) Ji Y., Chakraborty T., Wegner S. V., ACS Nano 2023, 17, 8992. - PMC - PubMed
-
- a) Westensee I. N., de Dios Andres P., Städler B., Adv. Mater. Technol. 2024, 9, 2301804;
- b) Adamala K. P., Dogterom M., Elani Y., Schwille P., Takinoue M., Tang T. Y. D., Nat. Rev. Mol. Cell Biol. 2024, 25, 162; - PubMed
- c) Guindani C., da Silva L. C., Cao S., Ivanov T., Landfester K., Angew. Chem., Int. Ed. 2022, 61, e202110855. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

