Gut microbiome-driven regulation of sex hormone homeostasis: a potential neuroendocrine connection
- PMID: 40071861
- PMCID: PMC11913384
- DOI: 10.1080/19490976.2025.2476562
Gut microbiome-driven regulation of sex hormone homeostasis: a potential neuroendocrine connection
Abstract
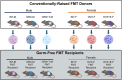
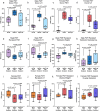
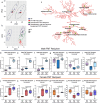
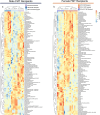
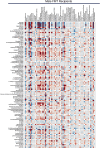
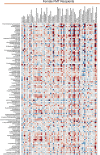
The gut microbiome is known to have a bidirectional relationship with sex hormone homeostasis; however, its role in mediating interactions between the primary regulatory axes of sex hormones and their productions is yet to be fully understood. We utilized both conventionally raised and gnotobiotic mouse models to investigate the regulatory role of the gut microbiome on the hypothalamic-pituitary-gonadal (HPG) axis. Male and female conventionally raised mice underwent surgical modifications as follows: (1) hormonally intact controls; (2) gonadectomized males and females; (3) gonadectomized males and females supplemented with testosterone and estrogen, respectively. Fecal samples from these mice were used to colonize sex-matched, intact, germ-free recipient mice through fecal microbiota transplant (FMT). Serum gonadotropins, gonadal sex hormones, cecal microbiota, and the serum global metabolome were assessed. FMT recipients of gonadectomized-associated microbiota showed lower circulating gonadotropin levels than recipients of intact-associated microbiota, opposite to that of FMT donors. FMT recipients of gonadectomized-associated microbiota also had greater testicular weights compared to recipients of intact-associated microbiota. The gut microbiota composition of recipient mice differed significantly based on the FMT received, with the male microbiota having a more concerted impact in response to changes in the HPG axis. Network analyses showed that multiple metabolically unrelated pathways may be involved in driving differences in serum metabolites due to sex and microbiome received in the recipient mice. In sum, our findings indicate that the gut microbiome responds to the HPG axis and subsequently modulates its feedback mechanisms. A deeper understanding of interactions between the gut microbiota and the neuroendocrine-gonadal system may contribute to the development of therapies for sexually dimorphic diseases.
Keywords: Gut microbiota; endocrine system; estrogen; sex hormones; testosterone.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
