Vaccine effectiveness dynamics against influenza and SARS-CoV-2 in community-tested patients in France 2023-2024
- PMID: 40071892
- PMCID: PMC11980197
- DOI: 10.1080/22221751.2025.2466699
Vaccine effectiveness dynamics against influenza and SARS-CoV-2 in community-tested patients in France 2023-2024
Abstract
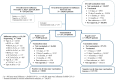
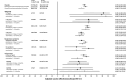
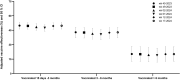
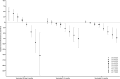
The epidemiology of respiratory viruses and vaccine effectiveness (VE) in the community are not well described. This study assessed VE against a positive test of influenza (VEf) and SARS-CoV-2 (VECov). Data from networks of community-based laboratories in France were collected during standard of care in the 2023-2024 epidemic season (n = 511,083 multiplex RT-PCR tests). Patients' demographics and symptoms were reported in addition to viral sequencing results. The test-negative design was used to estimate VEf and VECov by time since vaccination and calendar week. Adjusted VEf by age, sex, presence of symptoms, PCR technique, and week of testing, was 47.6% (95% CI: 44.3-50.7%). VEf was lower in patients ≥65 years (42.0%; 95% CI: 36.6-46.9%) than in 18-64 years (52.9%; 95% CI: 48.6-56.8%). The adjusted VEf against type A influenza, which represented 98% of typed viruses, was 51% (45%-56.6%) for patients vaccinated 15 days to 3 months before testing, and 35.5% (24.2%-45.3%) for those vaccinated 3-6 months before testing. For VECov, the adjusted estimate in patients vaccinated 15 days to 3 months prior to testing was 40.6% (7.2%-58.6%) at week 39, 24.8% (4.0%-38.8%) at week 45, and dropped systematically through the epidemic season as the JN.1 variant became dominant. This study showed moderate VEf and VECov against infection in the community and highlighted the impact of time since vaccination and age for both estimates, and the new variant emergence on VECov. These findings should be considered in future vaccination campaigns.
Keywords: COVID-19; Community testing; Effectiveness; Influenza; Vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- World Health Organization . European Respiratory Virus Surveillance Summary [Internet]. 2024. Available from: https://erviss.org/.
-
- Maurel M, Howard J, Kissling E, Pozo F, Pérez-Gimeno G, Buda S, et al. . Interim 2023/24 influenza A vaccine effectiveness: VEBIS European primary care and hospital multicentre studies, September 2023 to January 2024. Euro Surveill. 2024;29(8):2400089. doi:10.2807/1560-7917.ES.2024.29.8.2400089 - DOI - PMC - PubMed
-
- Skowronski DM, Zhan Y, Kaweski SE, Sabaiduc S, Khalid A, Olsha R, et al. . 2023/24 mid-season influenza and omicron XBB.1.5 vaccine effectiveness estimates from the Canadian Sentinel Practitioner Surveillance Network (SPSN). Euro Surveill. 2024;29(7):2400076. doi:10.2807/1560-7917.ES.2024.29.7.2400076 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
