Development of Antibody-Drug Conjugates for Malignancies of the Uterine Corpus: A Review
- PMID: 40072062
- PMCID: PMC11898814
- DOI: 10.3390/cells14050333
Development of Antibody-Drug Conjugates for Malignancies of the Uterine Corpus: A Review
Abstract
Despite recent advances in cancer treatment, the prognosis for uterine malignancies (carcinoma and sarcoma) requires further improvement. Antibody-drug conjugates (ADCs) have emerged as a novel class of anti-cancer therapeutic agents, and multiple ADCs have been approved for other types of cancer. In 2024, trastuzumab deruxtecan received approval from the US Food and Drug Administration for cancer types and became the first ADC approved for the treatment of uterine malignancies. Many ADCs are currently being investigated in uterine malignancies, and therefore, there is a need to gain a deeper understanding of ADCs. In this article, we aim to provide a comprehensive overview of the advancements in ADCs. The contents of this article include the structure and mechanism of action, an analysis of recent clinical trials, and expected future clinical questions. This article also focuses on uterine sarcoma, which is not often highlighted as a target for ADC treatment.
Keywords: AXL; B7-H3; B7-H4; FR?; HER2; TROP2; antibody–drug conjugate; endometrial carcinoma; uterine carcinosarcoma; uterine sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
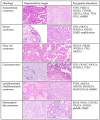

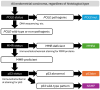
References
-
- Colombo N., Biagioli E., Harano K., Galli F., Hudson E., Antill Y., Choi C.H., Rabaglio M., Marme F., Marth C., et al. Atezolizumab and chemotherapy for advanced or recurrent endometrial cancer (AtTEnd): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024;25:1135–1146. doi: 10.1016/S1470-2045(24)00334-6. - DOI - PubMed
-
- Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., De Jesus-Acosta A., Delord J.P., Geva R., Gottfried M., Penel N., Hansen A.R., et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

