Skim Milk Culture of Lactobacillus johnsonii SBT0309 Increases Intestinal Alkaline Phosphatase Activity and Inhibits Lipopolysaccharide-Induced Interleukin-8 Production in Intestinal Epithelial Cells
- PMID: 40072089
- PMCID: PMC11898809
- DOI: 10.3390/cells14050358
Skim Milk Culture of Lactobacillus johnsonii SBT0309 Increases Intestinal Alkaline Phosphatase Activity and Inhibits Lipopolysaccharide-Induced Interleukin-8 Production in Intestinal Epithelial Cells
Abstract
Background/objectives: Intestinal alkaline phosphatase (IAP) is an enzyme expressed in the intestinal brush border, which may exert anti-inflammatory effects by detoxifying lipopolysaccharides (LPSs), thereby preventing metabolic disorders. Various food components have been reported to influence IAP activity. However, few studies have evaluated the effects of fermented milk on IAP activity. In this study, we aimed to investigate fermented milk with high IAP-activating capacity and investigate its effect.
Methods: We screened a skim milk culture (SC), a fermented milk model, using differentiated Caco-2 cells. We investigated the effect of SC on IAP activity and gene expression in the Drosophila midgut. Quantitative PCR and immunoblot assays were conducted to examine gene and protein levels.
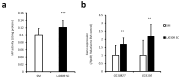
Results: Among the SC samples from different lactic acid bacteria or bifidobacteria, the SC of Lactobacillus johnsonii SBT0309 (LJ0309 SC) demonstrated a particularly strong capacity to activate IAP in Caco-2 cells, demonstrated by significantly increased IAP gene expression and protein levels in Caco-2 cells. Additionally, LJ0309 SC inhibited increased secretion of IL-8 in LPS-stimulated Caco-2 cells. Finally, in Drosophila melanogaster fed LJ0309 SC, we observed an increase in both IAP activity and gene expression in the midgut.
Conclusions: LJ0309 SC increased IAP activity and gene expression in both Caco-2 cells and the Drosophila midgut, and inhibited the inflammatory response in LPS-stimulated Caco-2 cells. Although further in vivo studies are required, LJ0309 SC might help to ameliorate LPS-induced inflammation and disease via IAP activation.
Keywords: Caco-2 cells; Drosophila melanogaster; Lactobacillus johnsonii SBT0309; fermented milk; intestinal alkaline phosphatase; lipopolysaccharide; skim milk culture.
Conflict of interest statement
All authors are employed by MEGMILK SNOW BRAND Co., Ltd. The results of this study were neither influenced nor constrained by this fact.
Figures



References
-
- Alam S.N., Yammine H., Moaven O., Ahmed R., Moss A.K., Biswas B., Muhammad N., Biswas R., Raychowdhury A., Kaliannan K., et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann. Surg. 2014;259:715–722. doi: 10.1097/SLA.0b013e31828fae14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

