Podocyte A20/TNFAIP3 Controls Glomerulonephritis Severity via the Regulation of Inflammatory Responses and Effects on the Cytoskeleton
- PMID: 40072109
- PMCID: PMC11898495
- DOI: 10.3390/cells14050381
Podocyte A20/TNFAIP3 Controls Glomerulonephritis Severity via the Regulation of Inflammatory Responses and Effects on the Cytoskeleton
Abstract
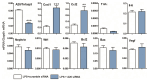
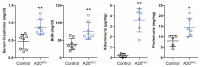
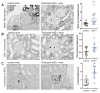
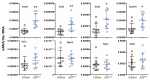
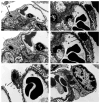
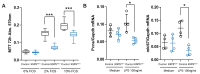
A20/Tnfaip3, an early NF-κB response gene and key negative regulator of NF-κB signaling, suppresses proinflammatory responses. Its ubiquitinase and deubiquitinase activities mediate proteasomal degradation within the NF-κB pathway. This study investigated the involvement of A20 signaling alterations in podocytes in the development of kidney injury. The phenotypes of A20Δpodocyte (podocyte-specific knockout of A20) mice were compared with those of control mice at 6 months of age to identify spontaneous changes in kidney function. A20Δpodocyte mice presented elevated serum urea nitrogen and creatinine levels, along with increased accumulation of inflammatory cells-neutrophils and macrophages-within the glomeruli. Additionally, A20Δpodocyte mice displayed significant podocyte loss. Ultrastructural analysis of A20 podocyte-knockout mouse glomeruli revealed hypocellularity of the glomerular tuft, expansion of the extracellular matrix, podocytopenia associated with foot process effacement, karyopyknosis, micronuclei, and podocyte detachment. In addition to podocyte death, we also observed damage to intracapillary endothelial cells with vacuolation of the cytoplasm and condensation of nuclear chromatin. A20 expression downregulation and CRISPR-Cas9 genome editing targeting A20 in a podocyte cell line confirmed these findings in vitro, highlighting the significant contribution of A20 activity in podocytes to glomerular injury pathogenesis. Finally, we analyzed TNFAIP3 transcription levels alongside genes involved in apoptosis, anoikis, NF-κB regulation, and cell attachment in glomerular and tubular compartments of kidney biopsies of patients with various renal diseases.
Keywords: A20; Tnfaip3; anoikis; inflammation; podocyte.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

