Sunlight-Induced Photocatalytic Removal of Paracetamol Using Au-TiO2 Nanoparticles
- PMID: 40072161
- PMCID: PMC11901493
- DOI: 10.3390/nano15050358
Sunlight-Induced Photocatalytic Removal of Paracetamol Using Au-TiO2 Nanoparticles
Abstract
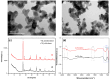
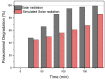
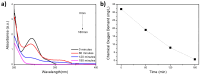
Using sunlight as the driving force for photocatalytic processes holds great promise for sustainability. As a starting point for developing a material capable of degrading aquatic pollutants using solar energy as a stimulus, this work focuses on synthesizing Au-TiO2 nanocomposites using the deposition-precipitation method. Characterization of Au-TiO2 nanoparticles was performed by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and Transmission Electron Microscopy (TEM). A model pollutant, paracetamol, was used to test the synergetic effect of Au (0.05 wt%) nanoparticles (NPs) with TiO2 on photocatalytic activity. The influence of the parameters pH, loading (0.4, 0.8, and 1 g/L), pollutant concentration (20, 30, 40 ppm), and contact time (30, 60, 90, 120, 150, and 180 min) was studied by exposing the NPs to solar radiation. The photocatalytic degradation was most effective at a contact time of 3 h, an initial concentration of 20 ppm, and a pH of 6.8. Under these conditions, paracetamol in 1 g/L of Au-TiO2 nanocomposites can be degraded by more than 99.17% under solar irradiation. As a result of the Au-TiO2 composite's ability to successfully serve as a photocatalyst using sun radiation, water purification processes can be more widespread, cost-effective, and environmentally friendly.
Keywords: Au-TiO2 catalyst; paracetamol; photocatalytic degradation; solar light; water remediation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bhatnagar A., Minocha A. Conventional and non-conventional adsorbents for removal of pollutants from water–A review. Indian J. Chem. Technol. 2006;13:203–217.
-
- Ashraf I., Agarwal A., Singh N.B. Nanotechnology to Monitor, Remedy, and Prevent Pollution. Elsevier; Amsterdam, The Netherlands: 2024. Removal of pharmaceuticals, cosmetics, and toiletries from water by nanomaterials; pp. 323–340.
LinkOut - more resources
Full Text Sources
Research Materials

