Antitumor efficacy of intermittent low-dose erlotinib plus sulindac via MHC upregulation and remodeling of the immune cell niche
- PMID: 40072251
- PMCID: PMC12079632
- DOI: 10.1002/ijc.35409
Antitumor efficacy of intermittent low-dose erlotinib plus sulindac via MHC upregulation and remodeling of the immune cell niche
Abstract
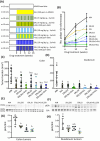
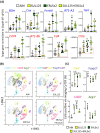
A previously reported clinical trial in familial adenomatous polyposis (FAP) patients treated with erlotinib plus sulindac (ERL + SUL) highlighted immune response/interferon-γ signaling as a key pathway. In this study, we combine intermittent low-dose ERL ± SUL treatment in the polyposis in rat colon (Pirc) model with mechanistic studies on tumor-associated immune modulation. At clinically relevant doses, short-term (16 weeks) and long-term (46 weeks) ERL ± SUL administration results in near-complete tumor suppression in Pirc colon and duodenum (p < 0.0001). We identify a low-dose threshold for significant antitumor activity in Pirc rats given SUL at 125 ppm in the diet plus ERL at 5 mg/kg body weight via twice-weekly oral gavage (SUL125 + ERL5 × 2). Longitudinal analyses show diminished expression of MHC class I and II genes in polyps larger than Grade 5, a novel finding in the Pirc model. Treatment with ERL ± SUL upregulates the corresponding MHC and immune-associated factors in a subset of Pirc colon polyps, Pirc tumor cell lines, murine colon carcinoma cells, and FAP patient-derived organoids, with Nlrc5 playing a critical role in this effect. Imaging mass cytometry reveals that SUL125 + ERL5 × 2 increases tumor-associated Cd4+ T cells by ~2.6-fold (p < 0.05), with no apparent effect on Cd8+ T cells. The treatment also increases tumor-associated Cd68+ cells (p < 0.05) and decreases Foxp3+ (p < 0.01) and Arg1+ (p < 0.05) cells. Thus, intermittent low-dose ERL + SUL treatment enhances tumor-associated MHC expression and remodels the immune cell niche toward a more permissive "helper" immune microenvironment. We conclude that early immune-interception strategies targeting interferon-γ signaling may benefit FAP patients at drug doses below the clinical standard of care.
Keywords: MHC; erlotinib; familial adenomatous polyposis (FAP); sulindac; tumor immunomodulation.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
E. Vilar has consulting/advisory roles with Janssen Research, Recursion Pharma, Guardant Health (Sept 2022), Rising Tide Foundation (May 2023), Nouscom, Abbvie, and research support from Janssen Research. P.H. Brown is a stockholder in GeneTex (less than 1%), which is unrelated to this work. The other authors declare no potential conflicts of interest.
Figures




References
-
- Lynch P. Chemoprevention of familial adenomatous polyposis. Fam Cancer. 2016;15:467‐475. - PubMed
MeSH terms
Substances
Grants and funding
- RO1 CA257559/Division of Cancer Prevention, National Cancer Institute
- 75N93019D00021/AO/NIAID NIH HHS/United States
- R01 CA257559/CA/NCI NIH HHS/United States
- T.O. 75N91019D00021/Division of Cancer Prevention, National Cancer Institute
- P30 CA016672/CA/NCI NIH HHS/United States
- 75N96024D00004/ES/NIEHS NIH HHS/United States
- 75N90024D00004/CL/CLC NIH HHS/United States
- T.O. 75N91019F00130/Division of Cancer Prevention, National Cancer Institute
- 75N99019D00021/OF/ORFDO NIH HHS/United States
- RO1 CA122959/Division of Cancer Prevention, National Cancer Institute
- 75N90019D00021/CL/CLC NIH HHS/United States
- R01 CA122959/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

