Long-term safety and effectiveness of fenfluramine in children and adults with Dravet syndrome
- PMID: 40072476
- PMCID: PMC12169399
- DOI: 10.1111/epi.18342
Long-term safety and effectiveness of fenfluramine in children and adults with Dravet syndrome
Abstract
Objective: We analyzed the long-term safety and effectiveness of fenfluramine (FFA) in patients with Dravet syndrome (DS) in an open-label extension (OLE) study after participating in randomized controlled trials (RCTs) or commencing FFA de novo as adults.
Methods: Patients with DS who participated in one of three RCTs or were 19 to 35 years of age and started FFA de novo were included. Key endpoints were: incidence of treatment-emergent adverse events (TEAEs) in the safety population, and median percentage change in monthly convulsive seizure frequency (MCSF) from the RCT baseline to end of study (EOS) in the modified intent-to-treat (mITT) population. Post hoc analyses compared effectiveness in patients on concomitant stiripentol (STP) vs those not taking STP, and assessed safety (TEAEs) and effectiveness (Clinical Global Impression-Improvement [CGI-I] scale ratings) in patients enrolled as adults.
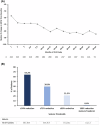
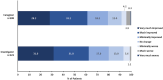
Results: A total of 374 patients, including 45 adults, received ≥1 FFA dose. Median FFA exposure was 824 days (range, 7-1280). TEAEs occurring in ≥10% of patients were pyrexia, nasopharyngitis, decreased appetite, seizure, decreased blood glucose, diarrhea, abnormal echocardiography (only physiologic regurgitation), upper respiratory tract infection, influenza, vomiting, and ear infection; no valvular heart disease or pulmonary arterial hypertension was observed over the OLE. In the mITT population (n = 324), median percentage change in MCSF from baseline to EOS was -66.8% (p < .001). The post hoc analyses of MCSF change from baseline to EOS in patients on concomitant STP (n = 75) was -36.2% vs -71.6% in those not on concomitant STP (n = 234) (p < .0001). In adult patients, 29 of 41 (70.7%) and 29 of 42 patients (69.1%) demonstrated clinically meaningful improvement on CGI-I at last visit as rated by caregivers and investigators, respectively.
Significance: Our OLE study of FFA in patients with DS confirmed previous positive findings and extended the exposure up to 3.5 years. No new or unexpected safety signals were observed and FFA demonstrated sustained and clinically meaningful reduction in MCSF.
Keywords: anti‐seizure medication; epilepsy; seizure.
© 2025 UCB, S.A and The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Ingrid E. Scheffer has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, GlaxoSmithKline, Knopp Biosciences, Nutricia, Rogcon, Takeda Pharmaceuticals, UCB, and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, LivaNova, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; has served as an investigator for Anavex Life Sciences, Cerebral Therapeutics, Cereval Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc., Epygenyx, ES‐Therapeutics, GW Pharma (now Jazz Pharmaceuticals), Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceutical, Zogenix (now a part of UCB), and Zynerba; has consulted for Atheneum Partners, Care Beyond Diagnosis, Epilepsy Consortium, Ovid Therapeutics, UCB and Zynerba Pharmaceuticals, Stoke Therapeutics, Praxis; and is a Non‐Executive Director of Bellberry Ltd. and a Director of the Australian Academy of Health and Medical Sciences. Rima Nabbout has received research funding from Eisai, GW Pharma (now Jazz Pharmaceuticals), Novartis, Shire, UCB, and Zogenix Inc. (now a part of UCB); is a consultant/advisor for Eisai, Biogen, GW Pharma (now Jazz Pharmaceuticals), Takeda, Novartis, Shire, and Zogenix Inc. (now a part of UCB); and a speaker for Advicenne, Eisai, BioMarin, GW Pharma (now Jazz Pharmaceuticals), Novartis, Nutricia, Neuraxpharma, and Zogenix Inc. (now a part of UCB) and UCB. Lieven Lagae has received grants from and is a consultant and/or speaker for Zogenix (now a part of UCB), LivaNova, UCB, Shire, Eisai, Novartis, Takeda/Ovid, NEL, and Epihunter. Orrin Devinsky has received Grant support from National Institute of Neurological Disorders and Stroke (NINDS), National Institute of Mental Health (NIMH), Multidisciplinary University Research Initiative (MURI), Centers for Disease Control and Prevention (CDC), and National Science Foundation (NSF); equity and/or compensation from Tilray, Receptor Life Sciences, Qstate Biosciences, Hitch Biosciences, Tevard Biosciences, Regel Biosciences, Script Biosciences, Actio Biosciences, Empatica, SilverSpike, and California Cannabis Enterprises (CCE); consulting fees from Zogenix (now a part of UCB), Ultragenyx, BridgeBio, GeneMedicine, and Marinus; patents for the use of cannabidiol in treating neurological disorders (owned by GW Pharmaceuticals, now Jazz Pharmaceuticals) for which he has waived any financial interests; other patents in molecular biology; and is managing partner of the PhiFundVentures. Stéphane Auvin has received personal fees from: Arvelle, Biocodex, GW Pharma (now Jazz Pharmaceuticals), and Xenon; personal fees and nonfinancial support from Biomarin, GW Pharma (now Jazz Pharmaceuticals), and Nutricia; personal fees/grants from Eisai and UCB for work as an investigator; and research support from Zogenix (now a part of UCB). Elizabeth A. Thiele has been a paid consultant for Zogenix (now a part of UCB), GW Pharma (now Jazz Pharmaceuticals), Upsher Smith, Aquestive; Research funding, GW Pharma (now Jazz Pharmaceuticals); Clinical trial site PI, GW Pharma (now Jazz Pharmaceuticals), and Zogenix (now a part of UCB). Elaine C. Wirrell has received consulting fees from Acadia, Amicus, Neurocrine, and Encoded Therapeutics. She also receives income from
Figures

References
-
- Zuberi SM, Wirrell E, Yozawitz E, Wilmshurst JM, Specchio N, Riney K, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1349–1397. - PubMed
-
- Aras LM, Isla J, Le Mingorance‐Meur A. The European patient with Dravet syndrome: results from a parent‐reported survey on antiepileptic drug use in the European population with Dravet syndrome. Epilepsy Behav. 2015;44:104–109. - PubMed
-
- Lagae L, Brambilla I, Mingorance A, Gibson E, Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60(1):63–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

