Biomarkers in gastroesophageal cancer 2025: an updated consensus statement by the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP)
- PMID: 40072752
- PMCID: PMC12399710
- DOI: 10.1007/s12094-025-03865-6
Biomarkers in gastroesophageal cancer 2025: an updated consensus statement by the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP)
Erratum in
-
Correction: Biomarkers in gastroesophageal cancer 2025: an updated consensus statement by the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP).Clin Transl Oncol. 2025 Sep;27(9):3833-3835. doi: 10.1007/s12094-025-03941-x. Clin Transl Oncol. 2025. PMID: 40580409 Free PMC article. No abstract available.
Abstract
Gastroesophageal carcinomas, including gastroesophageal adenocarcinoma (GEA) and esophageal squamous cell carcinoma (ESCC), pose a global health challenge due to their heterogeneity. The approach to diagnosis and treatment should first differentiate between GEA and ESCC. Over the past decade, therapies for metastatic or advanced GEA/ESCC have expanded, with several new therapeutic targets alongside trastuzumab for metastatic HER2-positive GEA. Four key biomarkers are essential for targeted therapy: HER2 overexpression/amplification, deficient mismatch repair/microsatellite instability (dMMR/MSI), PD-L1, and Claudin18.2 expression. Immunohistochemistry is the recommended method for these biomarkers evaluation. In addition, the assessment of biomarkers like FGFR2b is likely to become routine in the near future. Experts from the Spanish Society of Pathology (SEAP) and the Spanish Society of Medical Oncology (SEOM) have formed a consensus to optimize biomarker detection and usage in clinical practice. Their recommendations aim to improve personalized treatment strategies for GEA and ESCC patients, integrating new diagnostic insights into routine care.
Keywords: Biomarkers; Claudin 18.2; Gastroesophageal carcinoma; HER2; MSI/dMMR; PD-L1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: AMM has participated in consulting and/or advisory boards for Amgen, AstraZeneca, BeiGene, Jazz Pharmaceuticals, BMS, Novartis and MSD. Speaker honoraria from Astellas, Beigene, Jazz Pharmaceuticals. ICM has participated in consulting and/or advisory boards for BeiGene, BMS, Astellas, Roche, Gilead, AstraZeneca, and MSD. Speaker honoraria from Astellas, BMS, MSD, Merck, Daichii-Sankyo, Servier and Roche. TQA has participated in consulting and/or advisory boards of GSK, MSD and Roche. CMC has participated in consulting and/or advisory boards for BeiGene, BMS, Astellas, Gilead, and MSD. Speaker honoraria from Astellas, BMS, MSD, and Roche. JFP received honoraria for speakers' bureau participation, and serving on advisory boards from Astellas, AstraZeneca, Bristol-Myers Squibb (BMS), Esteve, Merck Sharp & Dohme (MSD), Novartis, Nutricia, Pfizer, Rovi, Takeda, and Viatris and research grants from Astellas, AstraZeneca, BMS, and MSD. RHF Consultant or Advisory Role: Roche, Merck-Serono, Amgen, MSD, BMS, Lilly, Celgene, Sanofi-Aventis, Servier, Astra-Zeneca, Bayer, Astellas, Organon. Research Funding: Roche, Merck-Serono, Amgen, MSD, Lilly, Celgene, Sanofi-Aventis, Servier, Bayer. Speaking: Roche, Merck-Serono, Amgen, MSD, BMS, Lilly, Celgene, Sanofi-Aventis, Servier, Bayer. Grant support: Amgen, MSD, BMS. Ethics approval and consent to participate: Not applicable. Research involving human participants and/or animals and informed consent: Not applicable. Consent for publication: Not applicable.
Figures

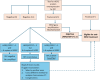
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. - PubMed
-
- Las cifras del cáncer en España. http://www.seom.org.
-
- Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):992–1004. - PubMed
-
- Ben-Aharon I, van Laarhoven HWM, Fontana E, Obermannova R, Nilsson M, Lordick F. Early-onset cancer in the gastrointestinal tract is on the rise-evidence and implications. Cancer Discov. 2023;13(3):538–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

