Using normative models pre-trained on cross-sectional data to evaluate intra-individual longitudinal changes in neuroimaging data
- PMID: 40072912
- PMCID: PMC11903031
- DOI: 10.7554/eLife.95823
Using normative models pre-trained on cross-sectional data to evaluate intra-individual longitudinal changes in neuroimaging data
Abstract
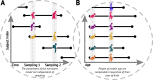
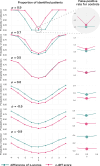
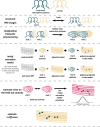
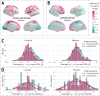
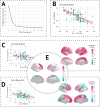
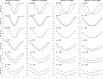
Longitudinal neuroimaging studies offer valuable insight into brain development, ageing, and disease progression over time. However, prevailing analytical approaches rooted in our understanding of population variation are primarily tailored for cross-sectional studies. To fully leverage the potential of longitudinal neuroimaging, we need methodologies that account for the complex interplay between population variation and individual dynamics. We extend the normative modelling framework, which evaluates an individual's position relative to population standards, to assess an individual's longitudinal change compared to the population's standard dynamics. Using normative models pre-trained on over 58,000 individuals, we introduce a quantitative metric termed 'z-diff' score, which quantifies a temporal change in individuals compared to a population standard. This approach offers advantages in flexibility in dataset size and ease of implementation. We applied this framework to a longitudinal dataset of 98 patients with early-stage schizophrenia who underwent MRI examinations shortly after diagnosis and 1 year later. Compared to cross-sectional analyses, showing global thinning of grey matter at the first visit, our method revealed a significant normalisation of grey matter thickness in the frontal lobe over time-an effect undetected by traditional longitudinal methods. Overall, our framework presents a flexible and effective methodology for analysing longitudinal neuroimaging data, providing insights into the progression of a disease that would otherwise be missed when using more traditional approaches.
Keywords: MRI; human; neuroimaging; neuroscience; normative modelling; psychosis; schizophrenia.
© 2024, Rehak Buckova et al.
Conflict of interest statement
BR, CF, RR, MK, CB, FŠ, JH No competing interests declared, AM Reviewing editor, eLife
Figures




Update of
- doi: 10.1101/2023.06.09.544217
- doi: 10.7554/eLife.95823.1
- doi: 10.7554/eLife.95823.2
- doi: 10.7554/eLife.95823.3
References
-
- Berthet P, Haatveit BC, Kjelkenes R, Worker A, Kia SM, Wolfers T, Rutherford S, Alnaes D, Dinga R, Pedersen ML, Dahl A, Fernandez-Cabello S, Dazzan P, Agartz I, Nesvåg R, Ueland T, Andreassen OA, Simonsen C, Westlye LT, Melle I, Marquand A. A 10-year longitudinal study of brain cortical thickness in people with first-episode psychosis using normative models. Schizophrenia Bulletin. 2024;51:95–107. doi: 10.1093/schbul/sbae107. - DOI - PMC - PubMed
-
- Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, Adler S, Alexopoulos GS, Anagnostou E, Areces-Gonzalez A, Astle DE, Auyeung B, Ayub M, Bae J, Ball G, Baron-Cohen S, Beare R, Bedford SA, Benegal V, Beyer F, Blangero J, Blesa Cábez M, Boardman JP, Borzage M, Bosch-Bayard JF, Bourke N, Calhoun VD, Chakravarty MM, Chen C, Chertavian C, Chetelat G, Chong YS, Cole JH, Corvin A, Costantino M, Courchesne E, Crivello F, Cropley VL, Crosbie J, Crossley N, Delarue M, Delorme R, Desrivieres S, Devenyi GA, Di Biase MA, Dolan R, Donald KA, Donohoe G, Dunlop K, Edwards AD, Elison JT, Ellis CT, Elman JA, Eyler L, Fair DA, Feczko E, Fletcher PC, Fonagy P, Franz CE, Galan-Garcia L, Gholipour A, Giedd J, Gilmore JH, Glahn DC, Goodyer IM, Grant PE, Groenewold NA, Gunning FM, Gur RE, Gur RC, Hammill CF, Hansson O, Hedden T, Heinz A, Henson RN, Heuer K, Hoare J, Holla B, Holmes AJ, Holt R, Huang H, Im K, Ipser J, Jack CR, Jr, Jackowski AP, Jia T, Johnson KA, Jones PB, Jones DT, Kahn RS, Karlsson H, Karlsson L, Kawashima R, Kelley EA, Kern S, Kim KW, Kitzbichler MG, Kremen WS, Lalonde F, Landeau B, Lee S, Lerch J, Lewis JD, Li J, Liao W, Liston C, Lombardo MV, Lv J, Lynch C, Mallard TT, Marcelis M, Markello RD, Mathias SR, Mazoyer B, McGuire P, Meaney MJ, Mechelli A, Medic N, Misic B, Morgan SE, Mothersill D, Nigg J, Ong MQW, Ortinau C, Ossenkoppele R, Ouyang M, Palaniyappan L, Paly L, Pan PM, Pantelis C, Park MM, Paus T, Pausova Z, Paz-Linares D, Pichet Binette A, Pierce K, Qian X, Qiu J, Qiu A, Raznahan A, Rittman T, Rodrigue A, Rollins CK, Romero-Garcia R, Ronan L, Rosenberg MD, Rowitch DH, Salum GA, Satterthwaite TD, Schaare HL, Schachar RJ, Schultz AP, Schumann G, Schöll M, Sharp D, Shinohara RT, Skoog I, Smyser CD, Sperling RA, Stein DJ, Stolicyn A, Suckling J, Sullivan G, Taki Y, Thyreau B, Toro R, Traut N, Tsvetanov KA, Tuulari JJ, Tzourio C, Vachon-Presseau É, Valdes-Sosa MJ, Valdes-Sosa PA, Valk SL, van Amelsvoort T, Vandekar SN, Vasung L, Victoria LW, Villeneuve S, Villringer A, Vértes PE, Wagstyl K, Wang YS, Warfield SK, Warrier V, Westman E, Westwater ML, Whalley HC, Witte AV, Yang N, Yeo B, Yun H, Zalesky A, Zar HJ, Zettergren A, Zhou JH, Ziauddeen H, Zugman A, Zuo XN, Bullmore ET, Alexander-Bloch AF, 3R-BRAIN. AIBL. Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Disease Repository Without Borders Investigators. CALM Team. Cam-CAN. CCNP. COBRE. cVEDA. ENIGMA Developmental Brain Age Working Group. Developing Human Connectome Project. FinnBrain. Harvard Aging Brain Study. IMAGEN. KNE96. Mayo Clinic Study of Aging. NSPN. POND. PREVENT-AD Research Group. VETSA Brain charts for the human lifespan. Nature. 2022;604:525–533. doi: 10.1038/s41586-022-04554-y. - DOI - PMC - PubMed
-
- Canal-Rivero M, Ruiz-Veguilla M, Ortiz-García de la Foz V, López-Díaz A, Garrido-Torres N, Ayesa-Arriola R, Vazquez-Bourgon J, Mayoral-van Son J, Brambilla P, Kircher T, Romero-García R, Crespo-Facorro B. Longitudinal trajectories in negative symptoms and changes in brain cortical thickness: 10-year follow-up study. The British Journal of Psychiatry. 2023;223:309–318. doi: 10.1192/bjp.2022.192. - DOI - PMC - PubMed
-
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, Chaarani B, Mejia MH, Hagler DJ, Daniela Cornejo M, Sicat CS, Harms MP, Dosenbach NUF, Rosenberg M, Earl E, Bartsch H, Watts R, Polimeni JR, Kuperman JM, Fair DA, Dale AM. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental Cognitive Neuroscience. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- 'BRAINCHART': 215698/Z/19/Z/WT_/Wellcome Trust/United Kingdom
- CZ.02.2.69/0.0/0.0/18_053/0017594/Ministry of Education, Youth and Sports
- 'BRADY' CZ.02.01.01/00/22_008/0004643/Programme Johannes Amos Comenius
- SGS22/062/OHK3/1 T/13/Czech Technical University Internal Grant Agency
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

