Modulating inhibitory synaptic plasticity to restore basal ganglia dynamics in Parkinson's disease
- PMID: 40073201
- PMCID: PMC12233548
- DOI: 10.1093/brain/awaf103
Modulating inhibitory synaptic plasticity to restore basal ganglia dynamics in Parkinson's disease
Abstract
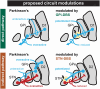
Parkinson's disease is characterized, in part, by hypoactivity of direct pathway inhibitory projections from striatum to the globus pallidus internus (GPi) and indirect pathway inhibitory projections from globus pallidus externus (GPe) to the subthalamic nucleus (STN). In people with Parkinson's disease (n = 32), we explored the potential use of intracranial stimulation for eliciting long-term potentiation (LTP) of these underactive pathways to produce improvement of symptoms that persist beyond stimulation cessation. During GPi deep brain stimulation (DBS) surgery, we found strong evidence (P < 0.05; BF10 > 10) of increased amplitudes of hand movements and striato-GPi evoked potentials before versus after high-frequency microstimulation. In a small sample of outpatients with sensing-enabled GPi-DBS, we found anecdotal evidence (P < 0.10; BF10 > 1) of improved hand movements and attenuated beta frequency oscillations. In STN, enduring behavioural effects, potentiation of GPe-STN projections (intraoperative), and decreases to beta oscillations (extraoperative) were not observed. Our findings support that LTP-like effects in GPi may produce motor improvements that extend beyond stimulation cessation, while the lack of effects in STN suggests the need for optimizing stimulation paradigms for effective LTP induction. These findings nevertheless highlight the potential of LTP-based strategies for sustained therapeutic benefits, which may be useful for mitigating DBS side-effects and optimizing battery usage.
Keywords: Parkinson’s disease; basal ganglia; deep brain stimulation; globus pallidus internus; subthalamic nucleus; synaptic plasticity.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
L.M. and W.D.H. hold intellectual property related to predicting and modelling neuronal responses to DBS (patent publication number: 20220152396; application number: 17/527,042). All other authors declare no competing interests related to this work.
Figures


References
-
- McGregor MM, Nelson AB. Circuit mechanisms of Parkinson’s disease. Neuron. 2019;101:1042–1056. - PubMed
-
- Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362:2077–2091. - PubMed
-
- Milosevic L, Gramer R, Kim TH, et al. Modulation of inhibitory plasticity in basal ganglia output nuclei of patients with Parkinson’s disease. Neurobiol Dis. 2019;124:46–56. - PubMed
-
- Precht W, Yoshida M. Blockage of caudate-evoked inhibition of neurons in the substantia nigra by picrotoxin. Brain Res. 1971;32:229–233. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

