Oral fluid supplementation for the prevention of post-dural puncture headache: A noninferiority randomized controlled trial
- PMID: 40073366
- PMCID: PMC11903041
- DOI: 10.1371/journal.pone.0319481
Oral fluid supplementation for the prevention of post-dural puncture headache: A noninferiority randomized controlled trial
Abstract
Aim(s): To investigate the impact of the absence of specific advice for oral fluid intake, compared to supplementation water intake on the occurrence of post-dural puncture headache.
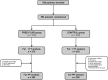
Design: A prospective, open-label, non-inferiority, multicenter trial including hospitalized patients requiring a diagnostic lumbar puncture in seven hospitals in France.
Methods: Patients were randomly allocated (1:1) either to receive no specific advice on oral fluid intake (FREE-FLUID), or to be encouraged to drink 2 liters of water (CONTROL) within the 2 hours after lumbar puncture. The primary outcome was the post-dural puncture headache rate within the 5 days after lumbar puncture, with a non-inferiority margin of 10%. The secondary outcome was the time-to-post-dural puncture headache onset between Day 0 and Day 5.
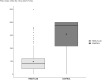
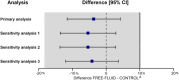
Results: From November 2016 and July 2019, we have included 554 participants. The primary outcomes occurs in 33.1% patients in the FREE-FLUID group, versus 38.0% in the CONTROL group with adjusted difference of 3.7%.
Conclusion: Among patients who had lumbar puncture, our study shows the noninferiority of the absence of specific advice on water intake after a lumbar puncture, compared with advice to increase oral fluid to prevent a post-dural puncture headache.
Impact: The value of questioning the appropriateness of non-evidence-based nursing care may allow time to be devoted to more relational and comforting care.
Reporting method: The study adheres to the CONSORT reporting guidelines.
Patient or public contribution: No patient or public contribution.
Trial registration: Clinical Trials.gov (NCT02859233, August 9, 2016).
Copyright: © 2025 Cartron et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

