Heme metabolism mediates RANKL-induced osteoclastogenesis via mitochondrial oxidative phosphorylation
- PMID: 40073838
- PMCID: PMC12103724
- DOI: 10.1093/jbmr/zjaf040
Heme metabolism mediates RANKL-induced osteoclastogenesis via mitochondrial oxidative phosphorylation
Abstract
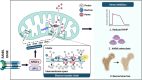
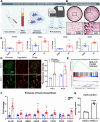
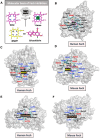
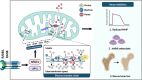
Bone undergoes life-long remodeling, in which disorders of bone remodeling could occur in many pathological conditions including osteoporosis. Understanding the cellular metabolism of osteoclasts (OCs) is key to developing new treatments for osteoporosis, a disease that affects over 200 million women worldwide per annum. We found that human OC differentiation from peripheral blood mononuclear cells derived from 8 female patients is featured with a distinct gene expression profile of mitochondrial biogenesis. Elevated mitochondrial membrane potential (MMP, Δψm) was also observed in receptor activator of NF-κB ligand (RANKL)-induced OCs. Interestingly, the gene pathways of heme synthesis and metabolism were activated upon RANKL stimulation, featured by transcriptomic profiling in murine cells at a single-cell resolution, which revealed a stepwise expression pattern of heme-related genes. The real-world human data also divulges potential links between heme-related genes and bone mineral density. Heme is known to have a role in the formation of functional mitochondrial complexes that regulate MMP. Disruption of heme biosynthesis via genetically silencing Ferrochelatase or a selective inhibitor, N-methyl Protoporphyrin IX (NMPP), demonstrated potent inhibition of OC differentiation, with a dose-dependent effect observed in NMPP treatment and a substantial efficacy even at a single dose. In vivo study further showed the protective effect of NMPP on ovariectomy-induced bone loss in female mice. Collectively, we found that RANKL-mediated signaling regulated mitochondrial formation and heme metabolism to synergistically support osteoclastogenesis. Inhibition of heme synthesis impaired OC formation and reversed excessive bone loss, representing a new therapeutic target for metabolic skeletal disorders.
Keywords: RANKL; heme; mitochondria; osteoclasts; osteoporosis.
Plain language summary
Bone remodeling is a lifelong process that can be disrupted in conditions like osteoporosis, a disease affecting over 200 million women globally per annum. Developing new treatments for osteoporosis requires understanding the cellular metabolism of osteoclasts (OCs), cells responsible for breaking down bone. In this study, we discovered that OC differentiation is characterized by increased mitochondrial biogenesis and elevated mitochondrial membrane potential (MMP). We also revealed that pathways involved in heme synthesis and metabolism were activated during this process, with real-world human data showing potential links between heme-related genes and bone mineral density. Heme is essential for forming functional mitochondrial complexes that regulate MMP and energy production, in which genetically knocking down heme catalyzing enzyme Ferrochelatase potently hampered receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis. We found that a selective inhibitor of heme biosynthesis, N-methyl Protoporphyrin IX, effectively inhibited OC differentiation in a dose-dependent manner and protected against bone loss in estrogen-deficiency mice. These findings suggest that RANKL-mediated signaling regulates mitochondrial formation and heme metabolism to support osteoclastogenesis. Inhibiting heme synthesis could be a novel therapeutic target for treating metabolic skeletal disorders like osteoporosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
None declared.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

