New routes for spermine biosynthesis
- PMID: 40074085
- PMCID: PMC11999265
- DOI: 10.1016/j.jbc.2025.108390
New routes for spermine biosynthesis
Abstract
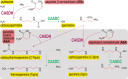
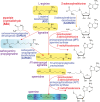
The polyamine spermine (Spm) is a flexible linear teraamine found in bacteria and eukaryotes and in all known cases is synthesized from triamine spermidine by addition of an aminopropyl group acquired from decarboxylated S-adenosylmethionine (dcAdoMet). We have now identified in bacteria a second biosynthetic route for Spm based on the formation of carboxyspermine from spermidine, dependent on aspartate β-semialdehyde (ASA). This route also produces thermospermine (Tspm) from spermidine via carboxythermospermine. Two enzymes, carboxyspermidine dehydrogenase and carboxyspermidine decarboxylase, are responsible for ASA-dependent production of spermidine, Spm, and Tspm from diamine putrescine. Production of Spm/Tspm from spermidine is controlled primarily by carboxyspermidine dehydrogenase, not carboxyspermidine decarboxylase. This new ASA-dependent Spm biosynthetic pathway is an example of convergent evolution, employing nonanalogous, nonhomologous enzymes to produce the same biosynthetic products as the dcAdoMet-dependent Spm pathway. We have also identified bacteria that encode hybrid Spm biosynthetic pathways dependent on both dcAdoMet and ASA. In the hybrid pathways, spermidine is produced from agmatine primarily by the ASA-dependent route, and Spm is synthesized from agmatine or spermidine by dcAdoMet-dependent modules. Both parts of the hybrid pathway initiate from agmatine and each produces N1-aminopropylagmatine, so that agmatine, N1-aminopropylagmatine, and spermidine are common, potentially shared metabolites. Bacteria such as Clostridium leptum that encode the hybrid pathway may explain the origin of Spm produced by the gut microbiota. This is the first example of convergent evolution of hybrid dcAdoMet- and ASA-dependent N1-aminopropylagmatine, spermidine, and Spm biosynthesis encoded in the same genomes and suggests additional polyamine biosynthetic diversification remains to be discovered.
Keywords: N(1)-aminopropylagmatine; aspartate β-semialdehyde; bacteria; biosynthesis; carboxyspermidine; carboxyspermine; convergent evolution; polyamine; spermidine; spermine; thermospermine.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interests with the contents of this article.
Figures







References
-
- Pegg A.E., Williams-Ashman H.G. Enzymic synthesis of spermine in rat prostate. Arch. Biochem. Biophys. 1970;137:156–165. - PubMed
-
- Raina A., Hannonen P. Separation of enzyme activities catalysing spermidine and spermine synthesis in rat brain. FEBS Lett. 1971;16:1–4. - PubMed
-
- Janne J., Schenone A., Williams-Ashman H.G. Separation of two proteins required for synthesis of spermidine from S-adenosyl-L-methionine and putrescine in rat prostate. Biochem. Biophys. Res. Commun. 1971;42:758–764. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

