ZNF667 alleviates the inflammatory damage in intervertebral disc degeneration via inhibiting NF-κB signaling pathway
- PMID: 40074310
- PMCID: PMC11897962
- DOI: 10.11817/j.issn.1672-7347.2024.240122
ZNF667 alleviates the inflammatory damage in intervertebral disc degeneration via inhibiting NF-κB signaling pathway
Abstract
Objectives: With the aging population, the incidence of intervertebral disc degeneration (IDD) is increasing every year. The pathogenesis of IDD is complex, and there are currently no effective treatment options. This study aims to investigate the specific function and underlying mechanism of zinc finger protein 667 (ZNF667) in the inflammatory damage of nucleus pulposus cells in IDD.
Methods: Differential expression genes (DEG) associated with IDD were screened from IDD-related datasets in the Gene Expression Omnibus (GEO) (GSE124272 and GSE150408), and ZNF667, which is closely related to gene transcriptional regulation, was selected and analyzed in several IDD-related datasets (GSE124272, GSE150408, GSE56081, GSE147383, GSE23130). Nucleus pulposus tissues were collected from 3 IDD patients and 3 trauma-induced vertebral fracture patients (serving as controls). Hematoxylin and eosin (HE) staining was performed for pathological examination, and immunohistochemistry (IHC) was used to assess ZNF667 expression in the nucleus pulposus tissues. Gene set enrichment analysis (GSEA) was then employed to elucidate the potential mechanisms of ZNF667. For in vitro validation, human primary nucleus pulposus cells were treated with 10 ng/mL of interleukin-1β (IL-1β) to establish an IDD cell model, and subsequently transfected with a ZNF667 overexpression plasmid. Flow cytometry was used to evaluate cell apoptosis, enzyme-linked immunosorbent assay (ELISA) measured the levels of inflammatory factors-cyclooxygenase-2 (COX-2), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the cell culture supernatant, real-time polymerase chain reaction (RT-PCR) quantified ZNF667 mRNA expression, and Western blotting assessed protein expression levels of ZNF667, myeloid differentiation factor 88 (MyD88), P65, and phosphorylated P65 (p-P65).
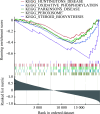
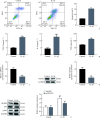
Results: Analysis of both the GEO datasets and clinical tissue samples revealed that ZNF667 expression is reduced in IDD. In IDD patients, the extracellular matrix and nucleus pulposus cells are significantly diminished, and the arrangement of fibrochondrocytes is disordered. GSEA results showed that ZNF667 may be involved in biological processes such as angiogenesis, epithelial-mesenchymal transition (EMT), oxidative phosphorylation, peroxisome function, steroid biosynthesis, and the NF-κB-mediated TNF-α signaling pathway. In vitro, ZNF667 was expressed at low levels in the IL-1β-induced IDD cell model, and overexpression of ZNF667 reversed the IL-1β-induced increase in cell apoptosis, the upregulation of inflammation factors (COX-2, IL-6, TNF-α), and the increased expression of NF-κB pathway-related proteins (MyD88 and the p-P65/P65 ratio) (all P<0.05).
Conclusions: ZNF667 can alleviate nucleus pulposus cell apoptosis and inflammatory responses by inhibiting the NF-κB signaling pathway, thereby exerting a protective effect on intervertebral discs. This finding not only provides new insights into the pathogenesis of IDD but also suggests a potential therapeutic target for its treatment.
目的: 随着老年化现象的加剧,椎间盘退变(intervertebral disc degeneration,IDD)的发病率逐年增加。IDD的发病机制复杂,目前尚无有效的治疗手段。本研究旨在深入探究在IDD特别是髓核细胞炎症损伤过程中锌指蛋白667(zinc finger protein 667,ZNF667)的具体功能及其作用机制。方法: 基于基因表达综合(gene expression omnibus,GEO)数据库中与IDD相关的数据集(GSE124272和GSE150408)筛选差异表达基因(differential expression genes,DEG),并将其中与基因转录调控密切相关的ZNF667作为目标基因。分析ZNF667基因在IDD相关数据集(GSE124272、GSE150408、GSE56081、GSE147383、GSE23130)中的表达情况。收集3例IDD患者和3例外伤致椎体骨折患者(作为对照)的髓核组织,采用苏木精-伊红(hematoxylin and eosin,HE)染色对髓核组织进行病理学检测,并通过免疫组织化学技术(immunohistochemistry technique,IHC)检测ZNF667在髓核组织中的表达水平。进一步通过基因富集分析(gene set enrichment analysis,GSEA)揭示ZNF667潜在的作用机制。为进行体外验证,采用10 ng/mL白细胞介素(interleukin-1β,IL-1β)处理人原代髓核细胞以构建IDD细胞模型,并进一步转染ZNF667过表达质粒至细胞;采用流式细胞术检测细胞凋亡情况,酶联免疫吸附分析(enzyme-linked immunosorbent assay,ELISA)检测细胞培养上清液中炎症因子环氧化酶-2(cyclooxygenase-2,COX-2)、白细胞介素-6(interleukin-6,IL-6)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)的水平,实时聚合酶链反应检测细胞中ZNF667的mRNA表达水平,蛋白质印迹法检测细胞中ZNF667、髓分化因子88(myeloid differentiation factor 88,MyD88)、P65和磷酸化P65(phosphorylation-P65,p-P65)的蛋白质表达水平。结果: 基于GEO数据库和临床组织样本检测的结果均表明ZNF667在IDD中低表达;IDD患者髓核组织细胞外基质和髓核细胞明显减少,纤维软骨细胞排列紊乱。GSEA结果显示ZNF667可能参与血管新生、上皮-间充质转化(epithelial-mesenchymal transition,EMT)、氧化磷酸化、过氧化物酶体、类固醇生物合成、核因子κB(nuclear factor-κB,NF-κB)介导的TNF-α信号通路等生物学过程。体外实验的结果进一步证实ZNF667在IL-1β诱导的IDD细胞模型细胞中低表达,且过表达ZNF667可逆转IL-1β导致的细胞凋亡率增加,炎症因子COX-2、IL-6、TNF-α水平上调和NF-κB信号通路相关的MyD88、p-P65/P65表达水平上调的趋势(均P<0.05)。结论: ZNF667可通过抑制NF-κB信号通路,减轻髓核细胞凋亡和炎症反应,发挥对椎间盘的保护作用。这一发现不仅为IDD的发病机制提供了新的视角,也为IDD的治疗提供了新的潜在靶点。.
Keywords: intervertebral disc degeneration; nuclear factor-κB signaling pathway; nucleus pulposus cells; zinc finger protein 667.
Conflict of interest statement
作者声称无任何利益冲突。
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

