Shared Pathophysiological Mechanisms and Genetic Factors in Early Menarche and Polycystic Ovary Syndrome
- PMID: 40074331
- PMCID: PMC11905354
- DOI: 10.1523/JNEUROSCI.1681-24.2024
Shared Pathophysiological Mechanisms and Genetic Factors in Early Menarche and Polycystic Ovary Syndrome
Abstract
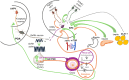
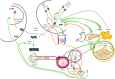
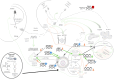
Early age at menarche (early AAM) and polycystic ovary syndrome (PCOS) are reproductive and metabolic disorders with overlapping pathophysiological and genetic features. Epidemiological studies suggest a link between these two conditions, both of which are characterized by dysregulation of the neuroendocrine pathways that control pulsatile gonadotropin-releasing hormone secretion, thus affecting gonadotropin release, particularly luteinizing hormone secretion. A common pathophysiology involving positive energy balance and abnormal metabolic status is evident in both disorders. Genetic and epigenetic factors influence the onset of puberty and reproductive outcomes. Genome-wide association studies have identified common genetic variants associated with AAM and PCOS, particularly in genes related to the neuroendocrine axis (e.g., FSHB) and obesity (e.g., FTO). In addition, high-throughput sequencing has revealed rare loss-of-function variants in the DLK1 gene in women with central precocious puberty (CPP), early menarche, and PCOS, who experienced adverse metabolic outcomes in adulthood. This review explores the shared pathophysiological mechanisms between CPP/early AAM and PCOS, examines potential genetic and epigenetic factors that may link these neuroendocrine reproductive conditions, and offers insights into future research and treatment strategies. Understanding these connections may provide new targets for therapeutic interventions and improve outcomes for individuals with these reproductive disorders.
Keywords: early menarche; genetic variants; metabolic outcomes; neuroendocrine pathways; polycystic ovary syndrome; precocious puberty.
Copyright © 2025 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
