Body weight reduction in women treated with tirzepatide by reproductive stage: a post hoc analysis from the SURMOUNT program
- PMID: 40074721
- PMCID: PMC12015656
- DOI: 10.1002/oby.24254
Body weight reduction in women treated with tirzepatide by reproductive stage: a post hoc analysis from the SURMOUNT program
Abstract
Objective: Increases in adiposity and adverse changes in adipose distribution commonly occur in women during midlife and with the onset of menopause. This post hoc analysis assessed body weight changes with tirzepatide by reproductive stage.
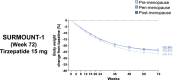
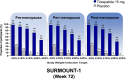
Methods: Women participants from SURMOUNT-1, -3, and -4 randomized to tirzepatide (15 mg or maximum tolerated dose) or placebo were retrospectively categorized as being in the pre-, peri-, or post-menopause stages. Body weight and waist circumference changes, the proportion of participants achieving body weight-reduction thresholds, and waist to height ratio (WHtR) category shift among those with baseline BMI < 35 kg/m2 were assessed at end of study treatment.
Results: In SURMOUNT-1, significantly greater body weight reductions from baseline were observed with tirzepatide versus placebo in women in the premenopause (26% vs. 2%), perimenopause (23% vs. 3%), and postmenopause stages (23% vs. 3%; p < 0.001). Greater waist circumference reductions were also observed with tirzepatide across the subgroups (22 vs. 4 cm, 20 vs. 5 cm, and 20 vs. 4 cm, respectively; p < 0.001). Across the reproductive stage subgroups, 97% to 98% of participants achieved body weight reductions that were ≥5% with tirzepatide versus 29% to 33% with placebo. Furthermore, 30% to 52% of women among the reproductive stage subgroups who had baseline BMI < 35 kg/m2 reached WHtR ≤ 0.49 (low central adiposity) with tirzepatide. Similar results were observed in SURMOUNT-3 and -4.
Conclusions: In this post hoc analysis, tirzepatide treatment was associated with significant body weight, waist circumference, and WHtR reductions versus placebo in women living with obesity or overweight and without type 2 diabetes, irrespective of reproductive stage.
© 2025 Eli Lilly and Company and The Author(s). Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society.
Conflict of interest statement
Beverly G. Tchang reports receiving consulting fees as an advisor for Novo Nordisk, Skye Bioscience, and Roman Health Ventures. Andreea Ciudin Mihai reports honoraria from AstraZeneca plc, Boehringer Ingelheim, Eli Lilly and Company, Esteve, and Novo Nordisk A/S for scientific talks, advisory board sessions, and attendance to congresses and is a member of the data monitoring committee for Boehringer Ingelheim clinical trials in obesity. Luis‐Emilio García‐Pérez, Adam Stefanski, Donna Mojdami, Irina Jouravskaya, Sirel Gurbuz, Rebecca Taylor, Chrisanthi A. Karanikas, and Julia P. Dunn are employees and shareholders of Eli Lilly and Company.
Figures


References
-
- Sternfeld B, Wang H, Quesenberry CP Jr, et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;160(9):912‐922. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

