ABO blood group system correlates with preoperative serum PSA level in patients with prostate cancer
- PMID: 40075227
- PMCID: PMC11904218
- DOI: 10.1038/s41598-025-93728-5
ABO blood group system correlates with preoperative serum PSA level in patients with prostate cancer
Abstract
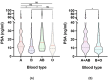
We previously demonstrated that the non-O blood type is associated with a higher risk of aggressive prostate cancer (PCa) than the O blood type. However, the influence of the ABO blood phenotype on the exact risk stratification parameters, especially prostate-specific antigen (PSA), remains unknown. We retrospectively evaluated 426 patients with PCa underwent radical prostatectomy in our centers. We divided them into three groups based on PSA levels: PSA1 (< 10 ng/ml), PSA2 (10-20 ng/ml), and PSA3 (> 20 ng/ml), and compared the clinicopathological characteristics among the groups. The association of clinicopathological parameters with PSA was determined using logistic regression analyses. The percentages of patients with advanced pathological stage T (pT) and grade, lymph node (pN) involvement, A + AB type, perineural invasion (PNI), vessel carcinoma embolus (VCE), and positive surgical margin rate were significantly higher in the PSA3 group than in the PSA1 group (all p < 0.05). Univariate logistic regression analyses revealed that type A + AB, grade, pT, pN, PNI, and VCE were positively correlated with PSA levels when comparing PSA3 with PSA1. Multivariate analysis showed that the association between blood type A + AB and PSA levels remained significant (OR 1.810, 95% CI 1.061-3.086, for PSA2 vs. PSA1; OR 2.682, 95% CI 1.550-4.641, for PSA3 vs. PSA1) after adjusting for confounding factors. The A + AB blood type was independently associated with PSA levels in PCa patients, indicating that the A-allele might participate in PSA synthesis and PSA screening may be more efficient for A-allele carriers.
Keywords: ABO blood system; Prostate cancer; Prostate-specific antigen; Radical prostatectomy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The studies involving humans were reviewed and approved by the Ethical Committees of Tsinghua University Affiliated Beijing Tsinghua Changgung Hospital and National Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. Due to the retrospective nature of the study, the Ethical Committees of Tsinghua University Affiliated Beijing Tsinghua Changgung Hospital waived the need of obtaining informed consent.
Figures
References
-
- Culp, M. B. et al. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol.77, 38–52 (2020). - PubMed
-
- Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol.79, 243–262 (2021). - PubMed
-
- Costello, A. J. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat. Rev. Urol.17, 177–188 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

