Transdiagnostic internet cognitive behavioural therapy for anxiety and depressive symptoms in postnatal women: protocol of a randomized controlled trial
- PMID: 40075340
- PMCID: PMC11905520
- DOI: 10.1186/s12888-025-06636-3
Transdiagnostic internet cognitive behavioural therapy for anxiety and depressive symptoms in postnatal women: protocol of a randomized controlled trial
Abstract
Background: Nearly 20% of women will be confronted with anxiety or depressive disorders during the perinatal period and this may lead to adverse outcomes for both mother and child. Cognitive behavioural therapy (CBT) is the psychological intervention with the most empirical support for the clinical management of anxiety and depressive disorders. Anxiety and depression frequently occur in women during the perinatal period, and there is growing evidence that internet-delivered CBT (iCBT) could be an acceptable and effective intervention. THIS WAY UP, an Australian digital mental health service, has developed a program for postnatal anxiety and depression. This study protocol aims to examine the acceptability and efficacy of a French-Canadian adaptation of the program.
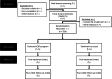
Methods/design: The research team propose to conduct a mixed hybrid type 1 pragmatic randomized clinical trial and implementation study to replicate the findings of the trial conducted in Australia by Loughnan et al. (2019), as well as explore barriers and facilitators to potential large-scale implementation. TREATMENT AND CONTROL CONDITIONS: a) postnatal anxiety and depression iCBT program with three lessons to complete in a six-week period, added to treatment-as-usual (TAU); b) TAU. Participants will include French-speaking women with probable postnatal depression or anxiety as per the Generalized Anxiety Disorder-7 (GAD-7) or the Edinburgh Postnatal Depression Scale (EPDS). The primary outcome measures will be the GAD-7 and the EPDS. Secondary outcome measures will comprise self-reported instruments to evaluate psychological distress, quality of life, mother-child experience, and treatment experience. Qualitative interviews with participants and health professionals will provide insights on acceptability and delivery of the iCBT program.
Statistical analysis: Statistical analysis will follow intent-to-treat principles. A mixed model regression approach will be used to account for between- and within-subject variations in the analysis of the effects of iCBT compared to TAU only intervention.
Discussion: The study will generate important data of efficacy and acceptability to patients, clinicians, and decision-makers to inform the scaling-up of the postnatal iCBT intervention in Canada.
Trial registration: ClinicalTrials.gov: NCT06778096, prospectively registered on 2025/01/16.
Keywords: Anxiety disorders; Depressive disorders; Non-guided internet cognitive behavioural therapy (iCBT); Postnatal; Pragmatic trial; Transdiagnostic; Web-based intervention.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol was approved by the ethics review boards of all study sites in Quebec, namely the Centre intégré universitaire de santé et de services sociaux de l’Estrie – Centre hospitalier universitaire de Sherbrooke (#MP-31–2024-5238; November 29th, 2023), the Centre intégré de santé et de services sociaux de la Montérégie-Est (#MP-31–2024-5238; March 27th, 2024), the Centre intégré de santé et de services sociaux de la Montérégie-Ouest (#RP-2023–12; October 30th, 2024) and the Centre intégré de santé et de services sociaux de la Montérégie-Centre (#MEO-31–2024-849; December 19th, 2024). For all phases of the study, written or verbal consent will be obtained. Ethics approval for Ontario to follow at a later date. Consent for publication: Not applicable. Competing interests: HMV is on the editorial board of BMC Psychiatry.
References
-
- Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 05 2017;210(5):315–323. 10.1192/bjp.bp.116.187179. - PubMed
-
- Falah-Hassani K, Shiri R, Dennis CL. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017;47(12):2041–53. 10.1017/S0033291717000617. - PubMed
-
- Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;09(219):86–92. 10.1016/j.jad.2017.05.003. - PubMed
-
- Bauer A, Knapp M, Parsonage M. Lifetime costs of perinatal anxiety and depression. J Affect Disord. 2016;192:83–90. 10.1016/j.jad.2015.12.005. - PubMed
-
- Highet N, Stevenson AL, Purtell C, Coo S. Qualitative insights into women’s personal experiences of perinatal depression and anxiety. Women Birth. 2014;27(3):179–84. 10.1016/j.wombi.2014.05.003. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical