Protective role of extracellular vesicles against oxidative DNA damage
- PMID: 40075425
- PMCID: PMC11905505
- DOI: 10.1186/s40659-025-00595-5
Protective role of extracellular vesicles against oxidative DNA damage
Abstract
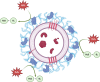
Background: Oxidative stress, a source of genotoxic damage, is often the underlying mechanism in many functional cell disorders. Extracellular vesicles (EVs) have been shown to be key regulators of cellular processes and may be involved in maintaining cellular redox balance. Herein, we aimed to develop a method to assess the effects of EVs on DNA oxidation using porcine seminal plasma extracellular vesicles (sEVs).
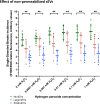
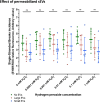
Results: The technique was set using a cell-free plasmid DNA to avoid the bias generated by the uptake of sEVs by sperm cells, employing increasing concentrations of hydrogen peroxide (H2O2) that generate DNA single-strand breaks (SSBs). Because SSBs contain a free 3'-OH end that allow the extension through quantitative PCR, such extension -and therefore the SYBR intensity- showed to be proportional to the amount of SSB. In the next stage, H2O2 was co-incubated with two size-differentiated subpopulations (small and large) of permeabilized and non-permeabilized sEVs to assess whether the intravesicular content (IC) or the surface of sEVs protects the DNA from oxidative damage. Results obtained showed that the surface of small sEVs reduced the incidence of DNA SSBs (P < 0.05), whereas that of large sEVs had no impact on the generation of SSBs (P > 0.05). The IC showed no protective effect against DNA oxidation (P > 0.05).
Conclusions: Our results suggest that the surface of small sEVs, including the peripheral corona layer, may exert a protective function against alterations that are originated by oxidative mechanisms. In addition, our in vitro study opens path to detect, localize and quantify the protective effects against oxidation of extracellular vesicles derived from different fluids, elucidating their function in physiopathological states.
Keywords: DNA fragmentation; DNA oxidation; Extracellular vesicles; Peripheral corona layer; Seminal plasma; Single-strand breaks.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experiments were approved under research codes CBE 367/2020 and CBE 538/2023 by the Bioethics Committee of the University of Murcia in its meetings of March 25, 2021, and June 16, 2023, respectively. Commercial semen samples were obtained from an artificial insemination center (AIM Iberica, Topigs Norsvin Spain SLU, Calasparra, Murcia, Spain), that fulfills with the European (ES13RS04P, July 2012) and Spanish (ES300130640127, August 2006) regulations for the commercialization of seminal AI-doses, and animal health and welfare. Consent for publication: Not applicable. Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be defined as a potential conflict of interest.
Figures




References
-
- Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. 10.1038/nrm.2017.125. - PubMed
-
- Waqas MY, Javid MA, Nazir MM, Niaz N, Nisar MF, Manzoor Z, Bhatti SA, Hameed S, Khaliq MH. Extracellular vesicles and exosome: insight from physiological regulatory perspectives. J Physiol Biochem. 2022;78:573–80. 10.1007/S13105-022-00877-6. - PubMed
-
- Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–59. 10.1038/S41565-021-00931-2. - PubMed
MeSH terms
Substances
Grants and funding
- PID2020-113493RB-I00/Ministerio de Ciencia, Innovación y Universidades
- PID2022-137738NA-I00/Ministerio de Ciencia, Innovación y Universidades
- RYC2021-034546-I/Ministerio de Ciencia, Innovación y Universidades
- 124/MTAI/22/Ministerio de Ciencia, Innovación y Universidades
- RYC2021-034764-I/Ministerio de Ciencia, Innovación y Universidades
LinkOut - more resources
Full Text Sources

