The role of lactate metabolism and lactylation in pulmonary arterial hypertension
- PMID: 40075458
- PMCID: PMC11905457
- DOI: 10.1186/s12931-025-03163-3
The role of lactate metabolism and lactylation in pulmonary arterial hypertension
Abstract
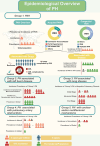
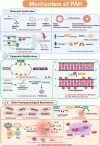
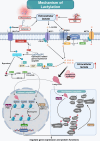
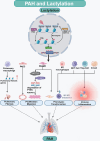
Pulmonary arterial hypertension (PAH) is a complex and progressive disease characterized by elevated pulmonary artery pressure and vascular remodeling. Recent studies have underscored the pivotal role of metabolic dysregulation and epigenetic modifications in the pathogenesis of PAH. Lactate, a byproduct of glycolysis, is now recognized as a key molecule that links cellular metabolism with activity regulation. Recent findings indicate that, in addition to altered glycolytic activity and dysregulated. Lactate homeostasis and lactylation-a novel epigenetic modification-also play a significant role in the development of PAH. This review synthesizes current knowledge regarding the relationship between altered glycolytic activity and PAH, with a particular focus on the cumulative effects of lactate in pulmonary vascular cells. Furthermore, lactylation, an emerging epigenetic modification, is discussed in the context of PAH. By elucidating the complex interplay between lactate metabolism and lactylation in PAH, this review aims to provide insights into potential therapeutic targets. Understanding these metabolic pathways may lead to innovative strategies for managing PAH and improving patient outcomes. Future research should focus on the underlying mechanisms through which lactylation influences the pathophysiology of PAH, thereby aiding in the development of targeted interventions.
Keywords: Glycolysis; Lactate; Lactylation; Protein translational modifications; Pulmonary arterial hypertension.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Ruopp NF, Cockrill BA. Diagnosis and treatment of pulmonary arterial hypertension: a review. JAMA. 2022;327:1379. - PubMed
-
- Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4:306–22. - PubMed
-
- Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension. Chest. 2010;137:376–87. - PubMed
-
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34-41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

