Injectable hydrogels with ROS-triggered drug release enable the co-delivery of antibacterial agent and anti-inflammatory nanoparticle for periodontitis treatment
- PMID: 40075491
- PMCID: PMC11900060
- DOI: 10.1186/s12951-025-03275-4
Injectable hydrogels with ROS-triggered drug release enable the co-delivery of antibacterial agent and anti-inflammatory nanoparticle for periodontitis treatment
Abstract
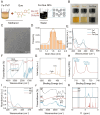
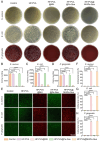
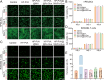
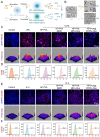
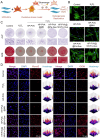
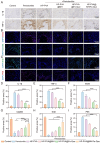
Periodontitis, a chronic inflammatory disease caused by bacteria, is characterized by localized reactive oxygen species (ROS) accumulation, leading to an inflammatory response, which in turn leads to the destruction of periodontal supporting tissues. Therefore, antibacterial, scavenging ROS, reducing the inflammatory response, regulating periodontal microenvironment, and alleviating alveolar bone resorption are effective methods to treat periodontitis. In this study, we developed a ROS-responsive injectable hydrogel by modifying hyaluronic acid with 3-amino phenylboronic acid (PBA) and reacting it with poly(vinyl alcohol) (PVA) to form a borate bond. In addition, the ROS-responsive hydrogel encapsulated the antibacterial agent minocycline hydrochloride (MH) and Fe-Quercetin anti-inflammatory nanoparticles (Fe-Que NPs) for on-demand drug release in response to the periodontitis microenvironment. This hydrogel (HP-PVA@MH/Fe-Que) exhibited highly effective antibacterial properties. Moreover, by modulating the Nrf2/NF-κB pathway, it effectively eliminated ROS and promoted macrophage polarization to the M2 phenotype, reducing inflammation and enhancing the osteogenic differentiation potential of human periodontal ligament stem cells (hPDLSCs) in the periodontal microenvironment. Animal studies showed that HP-PVA@MH/Fe-Que significantly reduced alveolar bone loss and enhanced osteogenic factor expression by killing bacteria and inhibiting inflammation. Thus, HP-PVA@MH/Fe-Que hydrogel had efficient antibacterial, ROS-scavenging, anti-inflammatory, and alveolar bone resorption-alleviation abilities, showing excellent application potential for periodontitis healing.
Keywords: Anti-inflammation; Antibacterial; Drug delivery; Periodontitis; ROS-responsive hydrogels.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the animal ethics committee of Southwest Medical University (Document No. 20230705-005). Consent for publication: All authors agree for publication. Competing interests: The authors declare no competing interests.
Figures






References
-
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. 10.1046/j.0906-6713.2002.003421.x. - PubMed
-
- Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, Webb S, Martin D, Genomics NN, Computational Biology C, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184:4090–e41044015. 10.1016/j.cell.2021.05.013. - PMC - PubMed
-
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. 10.1038/nrdp.2017.38. - PubMed
-
- Tonetti MS, Chapple ILC. Biological approaches to the development of novel periodontal therapies– Consensus of the seventh European workshop on periodontology. J Clin Periodontol. 2011;38:114–8. 10.1111/j.1600-051X.2010.01675.x. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

