Role of circulating tumor DNA in early-stage triple-negative breast cancer: a systematic review and meta-analysis
- PMID: 40075528
- PMCID: PMC11905660
- DOI: 10.1186/s13058-025-01986-y
Role of circulating tumor DNA in early-stage triple-negative breast cancer: a systematic review and meta-analysis
Abstract
Background: Triple-negative breast cancer (TNBC) accounts for 15% of all breast cancers and carries a worse prognosis relative to other breast cancer subtypes. This systematic review and meta-analysis evaluated the prognostic value of circulating tumor DNA (ctDNA) in early-stage TNBC.
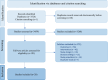
Methods: A literature search was conducted using Ovid Medline, Elsevier EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science Databases for publications up to 11/16/2023. Results were uploaded to Covidence and assessed by two independent reviewers. Studies assessing the use of ctDNA to predict recurrence free survival and related outcomes as well as overall survival were included. All recurrence outcomes were combined during analysis. Statistical analysis was performed using Revman Web. Log-hazard ratios (HR) were pooled for studies reporting recurrence and death as a time-to-event outcomes. Odds ratios (OR) were calculated and pooled for studies reporting patient-level data on recurrence, death, and pathological complete response (pCR). Prospero ID: CRD42023492529.
Results: A total of 3,526 publications were identified through our literature search, and 20 publications (n = 1202 patients) were included in the meta-analysis. In studies that reported recurrence as a time-to-event outcome, post-neoadjuvant (before or after surgery) ctDNA + status was associated with a higher likelihood of disease recurrence (HR 4.12, 95% confidence interval [CI] 2.81-6.04). For studies that reported patient-level data, post-neoadjuvant ctDNA + status was associated with higher odds of disease recurrence (OR 6.72, 95% CI 3.61-12.54). Pooled log-HR also revealed that ctDNA + status in the post-neoadjuvant setting (before or after surgery) was associated with worse overall survival (HR 3.26, 95% CI 1.88-5.63).
Conclusions: Our findings suggest that ctDNA could be used as a prognostic biomarker to anticipate the risk of relapse. However, it remains unclear if therapeutic intervention for patients who are ctDNA + can improve outcomes. While more studies are needed before incorporating ctDNA into clinical practice, the findings of this meta-analysis are reassuring and show the promise of ctDNA as a biomarker.
Keywords: Circulating tumor DNA; Disease free survival; Overall survival; Pathological complete response; Post-neoadjuvant; Prognosis; Recurrence-free survival; Triple-negative breast cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: Dr. Parsons has served in advisory roles for AstraZeneca, Caris, Daiichi-Sankyo, Natera, Neogenomics, SAGA Diagnostics, and Sermonix. Her institution has also received research funding from Merck outside of the submitted work. Dr. Medford has served as an advisor/consultant for AstraZeneca, Guardant Health, Illumina, Myriad Genetics, Natera, SAGA Diagnostics, and Science for America, outside the submitted work. Her research is supported by a National Institutes of Health K12 grant (K12CA087723). Dr. Huang reports grants, personal fees and non-financial support from AstraZeneca, Daiichi-Sankyo, EirGenix, Eli Lilly, Novartis, Pfizer, Roche; grants and non-financial support from MSD, grants and personal fees from Gilead, grants from OBI Pharma, grants from Seagen, and grants from Aston Sci outside the submitted work. Dr. Bidard has served in advisory role for AstraZeneca, Daiichi-Sankyo, Lilly, Novartis, Roche, Foresight Diagnostics, Menarini Silicon Biosystems, SAGA Diagnostics.The following authors do not have any conflicts of interest to disclose: DZ, SJ, JR, JD, FS, CW, PT, XH, MBasik, SR, PHL, LP, MBuss, IS.
Figures



References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A Cancer statistics. CA Cancer J Clin. 2023;73(1):17–48 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

