Cancer Risk in IBD Patients Treated with JAK Inhibitors: Reassuring Evidence from Trials and Real-World Data
- PMID: 40075582
- PMCID: PMC11899451
- DOI: 10.3390/cancers17050735
Cancer Risk in IBD Patients Treated with JAK Inhibitors: Reassuring Evidence from Trials and Real-World Data
Abstract
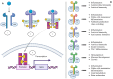
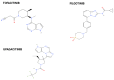
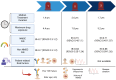
The advent of Janus kinase (JAK) inhibitors, including tofacitinib, filgotinib, and upadacitinib, has significantly widened the therapeutic options for patients with inflammatory bowel disease (IBD). These agents offer the advantage of oral administration and have demonstrated efficacy in inducing and maintaining remission. However, concerns regarding their safety have emerged, particularly concerning cardiovascular and infectious complications, which appear more pronounced in patients with pre-existing risk factors such as older age, smoking, or comorbidities. While these risks are better understood, the potential association between JAK inhibitors and malignancies remains a subject of ongoing investigation. Current data from randomised controlled trials, pooled and integrated analyses, and real-world studies provide conflicting evidence regarding cancer risk. Notably, studies in patients with rheumatologic diseases treated with JAK inhibitors have contributed additional insights into long-term safety outcomes. Despite the uncertainty surrounding malignancy risks, it is likely that predisposing factors, including older age, smoking history, and long-standing IBD with chronic inflammation, play a more substantial role in cancer development than JAK inhibitor therapy alone. This paper reviews safety data from clinical trials, meta-analyses, and observational studies, focusing on cancer risk in patients treated with JAK inhibitors for IBD. We also review evidence from rheumatology studies, highlighting the need for individualised risk assessment and close monitoring to optimise the safety profile of these medications in clinical practice.
Keywords: JAK inhibitors; cancer; filgotinib; inflammatory bowel disease; malignancies; tofacitinib; upadacitinib.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

