Absolute Quantification of Nucleotide Variants in Cell-Free DNA via Quantitative NGS: Clinical Application in Non-Small Cell Lung Cancer Patients
- PMID: 40075630
- PMCID: PMC11898635
- DOI: 10.3390/cancers17050783
Absolute Quantification of Nucleotide Variants in Cell-Free DNA via Quantitative NGS: Clinical Application in Non-Small Cell Lung Cancer Patients
Abstract
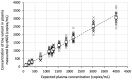
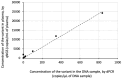
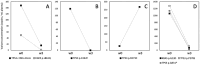
Background/Objectives: Circulating tumor DNA (ctDNA) analysis is a powerful tool for non-invasive monitoring of tumor burden and treatment response. Reliable quantification methods are critical for the effective use of ctDNA as a tumor biomarker. Digital PCR (dPCR) offers high sensitivity and quantification, but requires the prior knowledge of tumor-specific genomic alterations. Next-generation sequencing (NGS) provides a more comprehensive approach but is semi-quantitative, relying on variant allelic fraction (VAF), which can be influenced by non-tumor cell-free DNA. Methods: We developed a novel quantitative NGS (qNGS) method for absolute quantification of nucleotide variants, utilizing unique molecular identifiers (UMIs) and of quantification standards (QSs), short synthetic DNA sequences modified to include characteristic mutations for unique identification in sequencing data. We evaluated the performance of this method using plasma samples spiked with mutated DNA and plasma pools from cancer patients. We further applied our technique to plasma samples from four non-small cell lung cancer (NSCLC) patients enrolled in the ELUCID trial. Results: Our qNGS approach demonstrated robust linearity and correlation with dPCR in both spiked and patient-derived plasma samples. Notably, the analysis of clinical samples from the ELUCID trial revealed the ability of our method to simultaneously quantify multiple variants in a single plasma sample. Significant differences in ctDNA levels were observed between baseline and post-treatment samples collected after three weeks of front-line therapy. Conclusions: We introduce a novel qNGS method that enables the absolute quantification of ctDNA, independent of non-tumor circulating DNA variations. This technique was applied for the first time to serial samples from NSCLC patients, demonstrating its ability to simultaneously monitor multiple variants, making it a robust and versatile tool for precision oncology.
Keywords: NGS; NSCLC; ctDNA; dPCR; quantification.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Taus A., Camacho L., Rocha P., Hardy-Werbin M., Pijuan L., Piquer G., Lopez E., Dalmases A., Longaron R., Clave S., et al. Dynamics of EGFR Mutation Load in Plasma for Prediction of Treatment Response and Disease Progression in Patients With EGFR-Mutant Lung Adenocarcinoma. Clin. Lung Cancer. 2018;19:387–394 e382. doi: 10.1016/j.cllc.2018.03.015. - DOI - PubMed
-
- Herbreteau G., Vallee A., Knol A.C., Theoleyre S., Quereux G., Varey E., Khammari A., Dreno B., Denis M.G. Circulating Tumor DNA Early Kinetics Predict Response of Metastatic Melanoma to Anti-PD1 Immunotherapy: Validation Study. Cancers. 2021;13:1826. doi: 10.3390/cancers13081826. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

