The Complexity of Malignant Glioma Treatment
- PMID: 40075726
- PMCID: PMC11899524
- DOI: 10.3390/cancers17050879
The Complexity of Malignant Glioma Treatment
Abstract
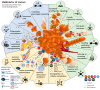
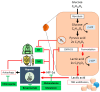
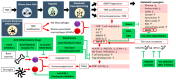
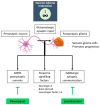
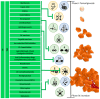
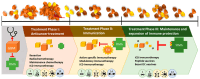
Malignant glioma is a highly aggressive, therapeutically non-responsive, and deadly disease with a unique tumor microenvironment (TME). Of the 14 currently recognized and described cancer hallmarks, five are especially implicated in malignant glioma and targetable with repurposed drugs: cancer stem-like cells, in general, and glioma stem-like cells in particular (GSCs), vascularization and hypoxia, metabolic reprogramming, tumor-promoting inflammation and sustained proliferative signaling. Each hallmark drives malignant glioma development, both individually and through interactions with other hallmarks, in which the TME plays a critical role. To combat the aggressive malignant glioma spatio-temporal heterogeneity driven by TME interactions, and to overcome its therapeutic challenges, a combined treatment strategy including anticancer therapies, repurposed drugs and multimodal immunotherapy should be the aim for future treatment approaches.
Keywords: immunotherapy; malignant glioma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

