Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma
- PMID: 40075744
- PMCID: PMC11898954
- DOI: 10.3390/cancers17050897
Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma
Abstract
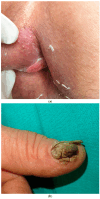
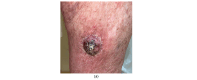
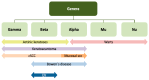
The human papillomavirus (HPV) has been associated with the carcinogenesis of cutaneous squamous cell carcinoma (cSCC), especially in immunosuppressed patients. This article reviews the microbiology of HPV and its role in tissue tropism, invasion, and oncogenesis. It also describes possible HPV oncogenic ability due to the inactivation of the host p53 and retinoblastoma protein (pRb) by HPV oncoproteins E6 and E7, producing a suppression of cell cycle checkpoints and uncontrolled cell proliferation that may eventually result in invasive carcinoma. We will focus on β-HPV types and their role in epidermodysplasia verruciformis (EV), as well as α types and their ability to cause cutaneous and mucosal pathology. We also intend to examine the clinical characteristics of cSCC related to HPV and host immunosuppression conditions such as solid organ transplant in order to provide management guidelines for patients with cSCC associated with HPV based on available data. Other topics addressed in this article include particular locations of cSCC, such as nails; the prognosis; the recurrence; therapeutic modalities; and the role of HPV vaccines.
Keywords: Bowen’s disease; cutaneous squamous cell carcinoma; epidermodysplasia verruciformis; human papillomavirus; keratoacanthoma; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

