A Rare Vitreoretinal Degenerative Disorder: Goldmann-Favre Syndrome Complicated with Choroidal Neovascularization in a Pediatric Patient
- PMID: 40075868
- PMCID: PMC11899382
- DOI: 10.3390/diagnostics15050622
A Rare Vitreoretinal Degenerative Disorder: Goldmann-Favre Syndrome Complicated with Choroidal Neovascularization in a Pediatric Patient
Abstract
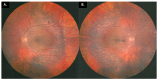
Goldmann-Favre syndrome (GFS) is a rare vitreoretinal degenerative disorder caused by mutations in the NR2E3 gene located on the short arm of chromosome 15. This condition, inherited in an autosomal recessive manner, was first described by Favre in two siblings, with Ricci later confirming its hereditary pattern. In GFS, rod photoreceptors are essentially replaced by S-cone photoreceptors. Enhanced S-Cone Syndrome (ESCS) and Goldmann-Favre syndrome are two distinct entities within the spectrum of retinal degenerative diseases, both caused by mutations in the NR2E3 gene. Despite sharing a common genetic basis, these conditions exhibit significantly different clinical phenotypes. ESCS is characterized by an excessive number of S-cones (blue-sensitive cones) with degeneration of rods and L-/M-cones, leading to increased sensitivity to blue light and early-onset night blindness. In contrast, GFS is considered a more severe form of ESCS, involving additional features such as retinal schisis, vitreous degeneration, and more pronounced visual impairment. GFS typically manifests in the first decade of life as night blindness (nyctalopia) and progressive visual acuity impairment. The clinical features include degenerative vitreous changes such as liquefaction, strands, and bands, along with macular and peripheral retinoschisis, posterior subcapsular cataract, atypical pigmentary dystrophy, and markedly abnormal or nondetectable electroretinograms (ERGs). Although peripheral retinoschisis is more common in GFS, central retinoschisis may also occur. Despite the consistent genetic basis, the phenotype of GFS can vary significantly among individuals. The differential diagnosis should consider diseases within the retinal degenerative spectrum, including retinitis pigmentosa, congenital retinoschisis, and secondary pigmentary retinopathy.
Keywords: Goldmann–Favre syndrome; autosomal recessive; choroidal neovascularization; vitreoretinal degenerative disorder.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Goldmann H. Présentation du rapport sur la biomicroscopie du du corps vitré et du fond d’oeil. Bull. Mem. Soc. Fr. d’Ophtalmol. 1957;70:265–272. - PubMed
LinkOut - more resources
Full Text Sources

