Growth Parameters and Growth-Related Hormone Profile in a Herd of Cattle up to 4 Years of Age Derived from Assisted Reproductive Technologies
- PMID: 40075913
- PMCID: PMC11898124
- DOI: 10.3390/ani15050631
Growth Parameters and Growth-Related Hormone Profile in a Herd of Cattle up to 4 Years of Age Derived from Assisted Reproductive Technologies
Abstract
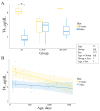
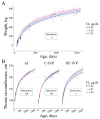
Assisted reproductive technologies (ART) are routinely used in livestock to generate animals of high genetic value. Despite representing an outstanding accomplishment, recent studies suggest differences in health, fertility, and gestational length of in vitro-produced compared to in vivo-derived animals. Currently, there are no data available on the long-term effects of ART on growth and development. This observational study aimed to understand the relationship between growth and growth-influencing hormones in a herd of cattle derived from artificial insemination (AI) or from in vitro-produced embryos either with BSA (C-IVP) or with reproductive fluids (RF-IVP) as a protein source in culture. Cortisol was associated positively with weight in AI and negatively with body length in males. Thyroxine decreased with age, and it was positively associated with thoracic circumference in RF-IVP. Insulin-like growth factor-1 was greater in RF-IVP than in C-IVP, and it was positively associated with body length and withers height. Growth hormone was greater in females than in males and RF-IVP compared to AI and C-IVP. In conclusion, we present here the first datasets on growth parameters and growth-influencing hormones in cattle from birth to 4 years of age without observing major evidence of differences depending on the embryo origin.
Keywords: animal health; assisted reproductive technologies; cattle; growth parameters; growth-related hormones; long-term impact.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Viana J.H. Statistics of Embryo Production and Transfer in Domestic Farm Animals. Embryo Technol. Newsl. 2022;40:22–40.
-
- Lafontaine S., Labrecque R., Blondin P., Cue R.I., Sirard M.A. Comparison of Cattle Derived from in Vitro Fertilization, Multiple Ovulation Embryo Transfer, and Artificial Insemination for Milk Production and Fertility Traits. J. Dairy Sci. 2023;106:4380–4396. doi: 10.3168/jds.2022-22736. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

