Removal and Recovery of AgNPs from Water by Sustainable Magnetic Nanoflocculants
- PMID: 40076147
- PMCID: PMC11902812
- DOI: 10.3390/polym17050650
Removal and Recovery of AgNPs from Water by Sustainable Magnetic Nanoflocculants
Abstract
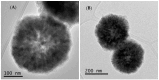
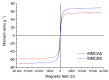
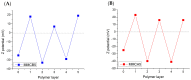
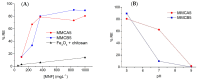
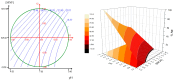
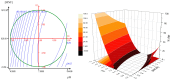
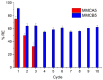
The presence of silver nanoparticles (AgNPs) in water bodies has emerged as a new environmental concern and the efficient separation of these nanoparticles remains a critical challenge. Here, we developed novel magnetic nanoflocculants for the recovery of AgNPs from water. Alternating layers of biopolymers, in particular, chitosan, alginate, and polymeric bio-based soluble substances (BBS) derived from urban waste, were coated on magnetic nanoparticles via the layer-by-layer technique to prepare reusable magnetic nanoflocculants (MNFs). The MNFs obtained were characterized with diverse physicochemical techniques. Surface response methodology, based on the Doehlert matrix, has shown to be a useful tool to determine the effect of pH (in the range 5-9), concentration of AgNPs (7-20 mg L-1), and MNFs (50-1000 mg L-1) on the performance of AgNPs removal. The model predicts a high AgNPs removal percentage at low pH values and high MNF concentration. In particular, for the most efficient MNFs, 90% of AgNPs removal was obtained at pH 5 and 600 mg L-1 MNF concentration. Additionally, the effects of AgNPs size, ionic strength, the presence of humic acids, and two types of surfactants (LAS anionic and TWEEN 20 nonionic) on the AgNPs removal were evaluated. Finally, recovery and reuse experiments showed that MNF made of Chitosan-BBS can be reused in ten cycles, losing only 30% of the initial removal capacity. Therefore, magnetic flocculation could represent a sustainable alternative for AgNPs separation with potential applications in water treatment and remediation of nanoparticle contamination.
Keywords: biopolymers; flocculants; magnetic nanomaterials; silver nanoparticles; water treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Temizel-Sekeryan S., Hicks A.L. Global Environmental Impacts of Silver Nanoparticle Production Methods Supported by Life Cycle Assessment. Resour. Conserv. Recycl. 2020;156:104676. doi: 10.1016/j.resconrec.2019.104676. - DOI
-
- StatNano Nanotechnology Products Database (NPD) [WWW Document] [(accessed on 30 August 2024)]. Available online: http://Product.Statnano.Com/
-
- Cleveland D., Long S.E., Pennington P.L., Cooper E., Fulton M.H., Scott G.I., Brewer T., Davis J., Petersen E.J., Wood L. Pilot Estuarine Mesocosm Study on the Environmental Fate of Silver Nanomaterials Leached from Consumer Products. Sci. Total Environ. 2012;421–422:267–272. doi: 10.1016/j.scitotenv.2012.01.025. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

