Synthetic Approaches to Novel DPP-IV Inhibitors-A Literature Review
- PMID: 40076268
- PMCID: PMC11902039
- DOI: 10.3390/molecules30051043
Synthetic Approaches to Novel DPP-IV Inhibitors-A Literature Review
Abstract
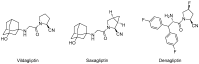
Dipeptidyl peptidase IV (DPP-IV) is a serine protease whose inhibition has been an object of considerable interest in the context of developing novel treatments for type 2 diabetes mellitus. The development of novel DPP-IV inhibitors from natural or synthetic origin has seen a growing scientific interest in recent years, especially during the SARS-CoV-2 pandemic, when DPP-IV inhibitors were found to be of beneficial therapeutic value for COVID-19 patients. The present manuscript aims to summarize the most recent information on the synthesis of different DPP-IV inhibitors, emphasizing the various heterocyclic scaffolds that can be found in them. Special attention is devoted to DPP-IV inhibitors that are currently in clinical trials. Different synthetic approaches for the construction of DPP-IV inhibitors are discussed, as well as the most recent developments in the field.
Keywords: DPP-IV inhibitors; drug design; heterocyclic synthesis; medicinal chemistry; type 2 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

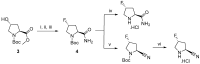
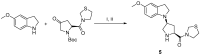



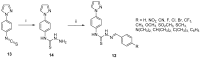












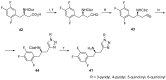

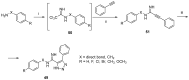








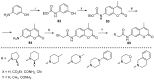
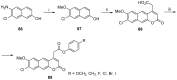























References
-
- Ahrén B., Simonsson E., Larsson H., Landin-Olsson M., Torgeirsson H., Jansson P.-A., Sandqvist M., Båvenholm P., Efendic S., Eriksson J.W., et al. Inhibition of Dipeptidyl Peptidase IV Improves Metabolic Control Over a 4-Week Study Period in Type 2 Diabetes. Diabetes Care. 2002;25:869–875. doi: 10.2337/diacare.25.5.869. - DOI - PubMed
-
- Penaforte-Saboia J.G., Couri C.E.B., Albuquerque N.V., Silva V.L.L., Olegario N.B.D.C., Fernandes V.O., Montenegro Junior R.M. Emerging Roles of Dipeptidyl Peptidase-4 Inhibitors in Delaying the Progression of Type 1 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2021;14:565–573. doi: 10.2147/DMSO.S294742. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

