A Study on the Oxidative Functionalization of a Poplar Biochar
- PMID: 40076273
- PMCID: PMC11901602
- DOI: 10.3390/molecules30051048
A Study on the Oxidative Functionalization of a Poplar Biochar
Abstract
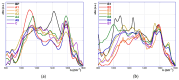
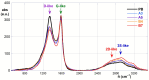
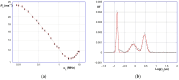
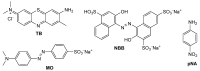
This study investigates the functionalization of a poplar biochar (PB), obtained by high-temperature pyrolysis, under oxidative conditions typically used in organic synthesis. In particular, concentrated nitric acid, a sulfonitric mixture and a piranha mixture were applied as oxidants at different temperatures and reaction times. In order to assess the outcome of the reaction conditions on the characteristics of the resultant products, these were characterized by a combination of imaging (SEM), spectroscopic (ATR-FTIR, RAMAN) and FFC-NMR relaxometric techniques. The latter techniques, rationalized in terms of the Kohlrausch-type stretched exponential kinetic model, were analyzed using a recently developed heuristic Monte Carlo method, providing insights into the water dynamics within material pore networks. Additionally, the water-holding capacity of the modified biochars and their abilities to adsorb some model dyes were evaluated. The results clarify the relationship between oxidative treatment conditions and biochar properties, highlighting their impact on both structural modifications and water dynamics within the porous network, and enabling us to identify the best reaction conditions for optimizing the features of the oxidized product.
Keywords: FFC-NMR relaxometry; adsorption; biochar; oxidation; piranha mixture; sulfonitric mixture.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Angelakis A.N., Snyder S.A. Wastewater Treatment and Reuse: Past, Present, and Future. Water. 2015;7:4887–4895. doi: 10.3390/w7094887. - DOI
-
- Salgot M., Folch M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health. 2018;2:64–74. doi: 10.1016/j.coesh.2018.03.005. - DOI
-
- Date M., Jaspal D. Dyes and heavy metals: Removal, recovery and wastewater reuse—A review. Sustain. Water Resour. Manag. 2024;10:90. doi: 10.1007/s40899-024-01073-8. - DOI
-
- Mary Ealias A., Meda G., Tanzil K. Recent Progress in Sustainable Treatment Technologies for the Removal of Emerging Contaminants from Wastewater: A Review on Occurrence, Global Status and Impact on Biota. Rev. Environ. Contamin. Toxicol. 2024;262:16. doi: 10.1007/s44169-024-00067-z. - DOI
-
- Suresh R., Rajendran S., Ponce L.C. Chapter 10—Waste-based adsorbents for the removal of emerging pollutants and their adsorption mechanisms. In: Hadi Dehghani M., Karri R.R., Tyagi I., editors. Sustainable Remediation Technologies for Emerging Pollutants in Aqueous Environment. Elsevier; Amsterdam, The Netherlands: 2024. pp. 203–221.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

