Spherical Amides with C3 Symmetry: Improved Synthetic Approach and Structural/Optical Analysis
- PMID: 40076300
- PMCID: PMC11901929
- DOI: 10.3390/molecules30051074
Spherical Amides with C3 Symmetry: Improved Synthetic Approach and Structural/Optical Analysis
Abstract
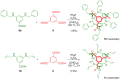
A spherical amide with C3 symmetry was synthesized by a one-step cyclization reaction using triphenylphosphine and hexachloroethane as coupling reagents. This method enabled synthesis of N-benzyl and N-allyl derivatives, which could not be obtained by the previously reported method. The optical resolution of each compound was measured, and their electronic circular dichroism spectra revealed that they were mirror images. The high structural symmetry resulted in a higher Δε (molar absorption difference against right or left circular polarization: εL - εR value compared to that of another structural isomer synthesized previously. The absolute structure of the enantiopure crystal of the N-benzyl derivative was determined using the Flack parameter obtained by X-ray crystallographic analysis.
Keywords: chiral macrocycle; cyclic amide; molecular scaffold; organic cage.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Gutsche C.D., Muthukrishnan R. Calixarenes. 1. Analysis of the Product Mixtures Produced by the Base-Catalyzed Condensation of Formaldehyde with Para-Substituted Phenols. J. Org. Chem. 1978;43:4905–4906. doi: 10.1021/jo00419a052. - DOI
-
- Alshahateet S.F., Altarawneh R.M., Al-Trawneh S.A., Al-Saraireh Y.M., Al-Tawarh W.M., Abuawad K.R., Abuhalaweh Y.M., Zerrouk M., Mansour A.A., Salghi R., et al. Cheminformatics-Based Design and Biomedical Applications of a New Hydroxyphenylcalix[4] Resorcinarene as Anti-Cancer Agent. Sci. Rep. 2024;14:30460. doi: 10.1038/s41598-024-82115-1. - DOI - PMC - PubMed
-
- Cram D.J., Cram Vol J.M., Cram D.J., Maxwell Cram J. Bridged Aromatic Compounds. Volume 20 Academic Press; Cambridge, MA, USA: 1959.
LinkOut - more resources
Full Text Sources
Miscellaneous

